Regulation of the Actin Cytoskeleton by Thrombin in Human Endothelial Cells: Role of Rho Proteins in Endothelial Barrier Function (original) (raw)
Abstract
Endothelial barrier function is regulated at the cellular level by cytoskeletal-dependent anchoring and retracting forces. In the present study we have examined the signal transduction pathways underlying agonist-stimulated reorganization of the actin cytoskeleton in human umbilical vein endothelial cells. Receptor activation by thrombin, or the thrombin receptor (proteinase-activated receptor 1) agonist peptide, leads to an early increase in stress fiber formation followed by cortical actin accumulation and cell rounding. Selective inhibition of thrombin-stimulated signaling systems, including Gi/o (pertussis toxin sensitive), p42/p44, and p38 MAP kinase cascades, Src family kinases, PI-3 kinase, or S6 kinase pathways had no effect on the thrombin response. In contrast, staurosporine and KT5926, an inhibitor of myosin light chain kinase, effectively blocked thrombin-induced cell rounding and retraction. The contribution of Rho to these effects was analyzed by using bacterial toxins that either activate or inhibit the GTPase. Escherichia coli cytotoxic necrotizing factor 1, an activator of Rho, induced the appearance of dense actin cables across cells without perturbing monolayer integrity. Accordingly, lysophosphatidic acid, an activator of Rho-dependent stress fiber formation in fibroblasts, led to reorganization of polymerized actin into stress fibers but failed to induce cell rounding. Inhibition of Rho with Clostridium botulinum exoenzyme C3 fused to the B fragment of diphtheria toxin caused loss of stress fibers with only partial attenuation of thrombin-induced cell rounding. The implication of Rac and Cdc42 was analyzed in transient transfection experiments using either constitutively active (V12) or dominant-interfering (N17) mutants. Expression of RacV12 mimicked the effect of thrombin on cell rounding, and RacN17 blocked the response to thrombin, whereas Cdc42 mutants were without effect. These observations suggest that Rho is involved in the maintenance of endothelial barrier function and Rac participates in cytoskeletal remodeling by thrombin in human umbilical vein endothelial cells.
INTRODUCTION
Thrombin is a tightly regulated serine proteinase that plays a central role in hemostasis. Via a widely expressed receptor, proteinase-activated receptor 1 (PAR-1) (Rasmussen et al., 1991; Vu et al., 1991), thrombin also coordinates tissue repair by inducing a host of cellular responses, including platelet aggregation, endothelial cell activation, leukocyte recruitment, and smooth-muscle cell growth (reviewed in Grand et al., 1996). After blood vessel injury, the endothelium becomes rapidly converted from a nonthrombogenic, noninflammatory contiguous surface to an adhesive surface with intercellular gaps that allow the passage of soluble plasma constituents and inflammatory cells from the vascular lumen to the underlying tissue. Thrombin has been found to potently stimulate this permeability transition of the endothelium both in vivo (Johnson et al., 1983; Lo et al., 1985) and in cultured endothelial cells (Garcia et al., 1995). In cultured cells, thrombin induces cell shape changes and gap formation in the monolayer (Laposata et al., 1983). Reorganization of the actin microfilament system is a major contributor to this effect. Accordingly, Phillips (1994) has found that phallacidin, an actin-stabilizing agent, prevents thrombin-induced increases in permeability of the endothelium to albumin.
Results from previous studies have suggested that the protein kinase C pathway plays a key role in regulating the endothelial barrier. Lynch et al. (1990) have shown that protein kinase C activation is correlated with increased 125I-albumin clearance rates in bovine pulmonary artery endothelial cells. However, evidence of protein kinase C involvement in thrombin-induced permeability increases relies largely on the use of kinase inhibitors. Finally, myosin light chain phosphorylation by the Ca2+/calmodulin-dependent myosin light chain kinase has been shown to be essential for retraction in thrombin-stimulated human umbilical vein endothelial cell (HUVEC) cultures (Wysolmerski and Lagunoff, 1990; Garcia et al., 1995; Goeckeler and Wysolmerski, 1995). Nonetheless, the signaling events that underlie distinct modifications of the actin cytoskeleton induced by thrombin in these cells have not been fully delineated.
The thrombin receptor is a seven-transmembrane receptor coupled to intracellular signaling systems via heterotrimeric GTP-binding proteins (see Van Obberghen-Schilling et al., 1995). Multiple G protein subtypes can be stimulated by the thrombin receptor in target cells including Gq, Gi/o, and G12 family proteins. A pertussis toxin–sensitive pathway has been implicated in cell growth stimulation (Chambard et al., 1987), linking the receptor to downstream events such as activation of Ras (Van Corven et al., 1993) and the p42/p44 MAP kinase module. Tyrosine phosphorylation of a number of proteins occurs in response to thrombin (Ferrell and Martin, 1988; Golden and Brugge, 1989), including adapter molecules such as Shc, which has been shown to participate in activation of the Ras/MAP kinase cascade (Chen et al., 1996). The tyrosine kinases focal adhesion kinase and Src family kinases are likely to be responsible for some of these events. Finally, thrombin modulates phospholipid metabolism and generation of key second messengers in target cells. Regulation of both phospholipases and lipid kinases by thrombin has been clearly demonstrated. Activation of Gq leads to stimulation of phospholipase C, intracellular Ca2+ mobilization, and activation of protein kinase C. In endothelial cells, activation of phospholipase A2 is considered the rate-limiting step in thrombin-induced prostaglandin synthesis (Hong and Deykin, 1982). Lipid kinases activated by thrombin include phosphatidylinositol (PI) 3-kinase and PI (4) 5-kinase. Interestingly, thrombin-stimulated PI 3-kinase and PI (4) 5-kinase activities were found to be regulated in part by the small GTP-binding protein Rho (Zhang et al., 1993, 1995; Chong et al., 1994). This finding is consistent with several recent observations that link lipid kinases and their phosphorylated products to regulation of the actin cytoskeleton (see Tapon and Hall, 1997).
The direct involvement of Rho family members, including Cdc42, Rac, and Rho, in regulating agonist-induced assembly of actin-based cytoskeletal structures was first demonstrated in Swiss 3T3 fibroblasts microinjected with dominant-interfering or constitutively active mutants (Ridley and Hall, 1992; Ridley et al., 1992; Nobes and Hall, 1995; Tapon and Hall, 1997). In neuronal cells, Jalink et al. (1994) showed that Rho is a major contributor to thrombin-induced neurite retraction and cell rounding. In the present study, we have reexamined the modifications of the actin cytoskeleton induced by thrombin in confluent endothelial cells and attempted to identify the biochemical mechanisms that regulate them. Our results based on the use of a variety of inhibitors (or activators) of known thrombin-stimulated signal transduction molecules rule out the requirement of protein kinase C, Ca2+ transients, Gi family proteins, PI 3-kinase, S6 kinase, and the p42/44/38 MAP kinase modules in thrombin-stimulated endothelial cell rounding and retraction. Furthermore, our observations using Rho-directed cytotoxins indicate that Rho does not control the inward-directed contractility observed in thrombin-treated endothelial cells. Rather, the Rho-dependent pathway controls cell flattening and barrier maintenance. Additional signaling pathways trigger actin polymerization beneath the plasma membrane (Rac dependent) and centripetal force generation (myosin light chain kinase dependent) required for the full effect of thrombin.
MATERIALS AND METHODS
Materials
Human α-thrombin (3209 NIH U/mg) was a generous gift of Dr. J.W. Fenton, II (New York State Department of Health, Albany, NY). The thrombin receptor peptide corresponding to the human receptor sequence (SFLLRN) was purchased from Neosystem (Strasbourg, France). The CP-118,556 (PP1) compound was kindly provided by Dr. S.B. Kadin (Pfizer, Groton, CT). Phorbol 12,13-dibutyrate (PdBu), histamine, staurosporine, genistein, DMSO, 8-bromo-cAMP, wortmannin, rapamycin, pertussis toxin, and FITC-phalloidin were from Sigma (St. Louis, MO). KT5926 was from Calbiochem (La Jolla, CA). GF 109203X was a kind gift from Dr. D. Toullec (Glaxo, Les Ulis, France) (Toullec et al., 1991). The Ca2+i chelator bis(2-aminophenoxy)ethane-_N,N,N′,N′_-tetra-acetic acid acetoxymethyl ester (BAPTA-AM) and Texas red–conjugated antibody to mouse immunoglobulin G were obtained from Molecular Probes (Eugene, OR). LY294002, PD98059, and SB203580 were purchased respectively from Biomol Research Laboratories (Plymouth, Meeting, PA), New England Biolabs (Beverly, PA), and SmithKline Beecham Pharmaceuticals (King of Prussia, PA). Platelet-activating factor (PAF) C18 (1-_O_-octadecyl-2-acetyl-sn-glycero-3-phosphocholine) and 1-oleoyl-lysophosphatidic acid (LPA) were from Alexis (San Diego, CA) and Avanti Polar Lipids (Alabaster, AL), respectively. Anti-c-Myc monoclonal antibody (9E10) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Bacterial Toxins
DC3B a fusion protein between Clostridium botulinum exoenzyme C3 and the B fragment of diphtheria toxin was purified to homogeneity as previously described (Aullo et al., 1993). Highly purified cytotoxic necrotizing factor 1 (CNF1) from uropathogenic Escherichia coli was obtained as described (Falzano et al., 1993).
Cells and Culture Conditions
Human vascular endothelial cells (HUVECs) were isolated from umbilical cord veins by collagenase perfusion as previously described (Barbieri et al., 1981). Cells were grown on 1.5% gelatin (Sigma)-coated 10-mm dishes in M199 medium (Life Technologies, Gaithersburg, MD) supplemented with 20% fetal calf serum, 30 μg/ml endothelial cell growth supplement from Sigma, 100 μg/ml heparin (Sigma), 50 U/ml penicillin, and 50 μg/ml streptomycin. Cells were used between the first and the fourth passages.
The human vascular endothelial cell line EA.hy926 was obtained from Dr. C.J. Edgell (University of North Carolina, Chapel Hill, NC) (Edgell et al., 1983). Cells were cultured in DMEM (Life Technologies) supplemented with 20% inactivated FCS and antibiotics. Cultures were maintained at 37°C in 5% CO2.
Plasmids and Transient Transfections
Plasmids pCMVCdc42V12, pCMVCdc42N17, pEXVRac1V12, and pEXVRac1N17 were kindly provided by Dr. M. Symons (Onyx Pharmaceuticals, Richmond, CA). pEGFP-N1 was from Clontech (Palo Alto, CA). One day after plating (4 × 105 cells/35-mm well), EA.hy926 cells were transfected with 1 μg plasmid DNA in presence of 6 μg Dac 30 (Eurogentec, Seraing, Belgium). Cells were fixed and stained for immunofluorescence 48 h later.
Localization of Actin Filaments and Immunofluorescence Studies
Human vascular endothelial cells were plated at a density of 2 × 105 cells per 17-mm2 Lab-Tek chamber slides (Poly Labo, Strasbourg, France) coated with fibronectin (7 μg/ml, Sigma) and grown to confluence. Two days later, cells were treated with the agonists in serum-free medium (EA.hy926 cells) or endothelial cell growth supplement–free medium (HUVEC) and fixed in a 3% paraformaldehyde/2% sucrose solution for 15 min at room temperature. After three washes in PBS, cells were permeabilized with 0.2% Triton X-100 (Sigma) for 3 min and stained. To localize F-actin filaments, cells were incubated with FITC-phalloidin (90 ng/slide) for 45–60 min at room temperature in a humid chamber. Myc-tagged Rac and Cdc42 mutants were detected using the monoclonal antibody 9E10 and a second antibody conjugated to Texas red. Fluorescence was observed with a Nikon (Garden City, NY) Diaphot fluorescence microscope.
Determination of Monolayer Permeability
Endothelial cells cultured up to confluence on fibronectin-coated Transwell filters (13 mm2, 3-μm pore size; Costar, Cambridge, MA) were used to assay the passage of macromolecules through the endothelial monolayer 5–6 d after seeding. Before the experiment, cells were incubated in serum-free growth medium containing 1% BSA for 1 h at 37°C. At the start of the experiment, agonists were added concomitantly with 1 mg/ml FITC-dextran (38,500 Da) to the upper compartment of the Transwell. After 15 min, samples were taken from the lower compartment and fluorescence (excitation at 492 nm and emission at 520 nm) was determined with a fluorimeter (LS-5 luminescence, spectrometer; Perkin Elmer-Cetus, Norwalk, CT). Results are expressed as the percentage of fluorescence obtained after treatment with 5 mM EGTA for 15 min.
RESULTS
Thrombin Induces Rounding of Human Endothelial Cells
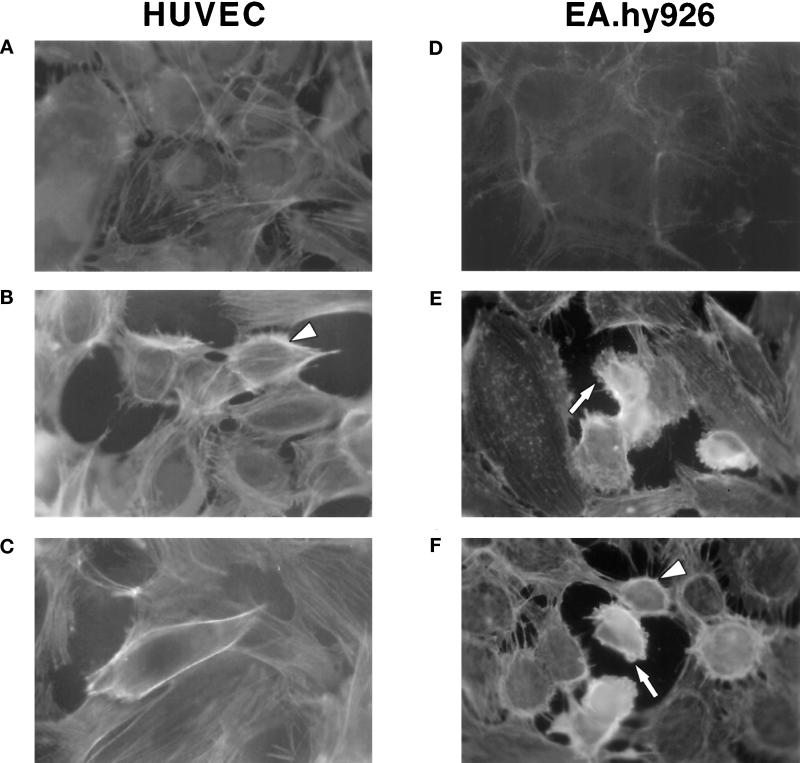
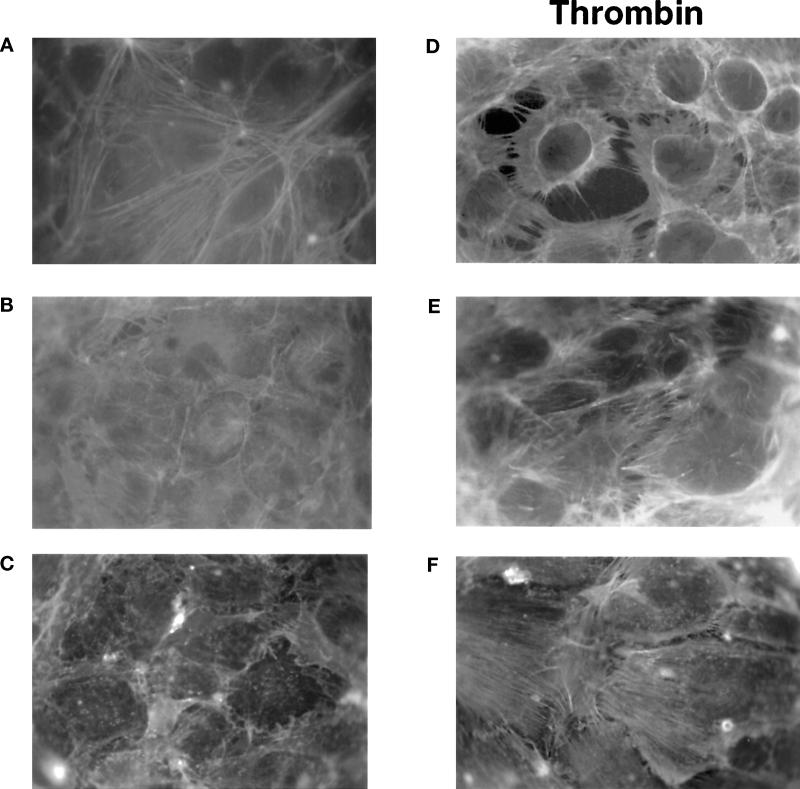
Confluent monolayers of HUVECs display a faint ring of polymerized actin at their periphery when stained with FITC-phalloidin and examined by fluorescence microscopy (Figure 1A). The addition of 10 nM thrombin to cells for 15 min causes a net increase in fluorescence intensity and a striking reorganization of the actin cytoskeleton (Figure 1B). The morphological changes are characterized by the disruption of cell–cell contacts, cell rounding, and the formation of gaps in the monolayer, as previously documented (Rabiet et al., 1996). This effect of thrombin is mediated by a cellular receptor, because the peptide agonist (designated TRP) of the proteinase-activated thrombin receptor (PAR-1) was found to elicit the same morphological changes as thrombin in HUVEC cultures, with maximal effects being obtained at 100 μM (Figure 1C). Rabiet et al. (1996) have reported that EA.hy926 cells, an established line of HUVECs (Edgell et al., 1983), are remarkably sensitive to the morphogenic action of thrombin. As shown in Figure 1D–F, changes in the actin cytoskeleton of EA.hy926 cells in response to thrombin or TRP are similar to those observed in HUVECs. Therefore, the analyses described below were performed both on this cell line and on HUVEC cultures.
Figure 1.
Thrombin receptor (PAR-1) activation induces cytoskeletal reorganization in human endothelial cell monolayers. Primary cultured HUVECs (left panel) or the HUVEC-derived cell line EA.hy926 (right panel) were grown to confluence and treated with medium alone (A and D), 10 nM thrombin (B and E), or 100 μM TRP (C and F) for 15 min at 37°C. Staining of the actin cytoskeleton was performed as described in MATERIALS AND METHODS. Arrowheads indicate the formation of a ring of peripheral actin, whereas arrows indicate the blebs that are present in some round cells. Photographs (magnification, 1000×) are representative of results from at least four independent experiments.
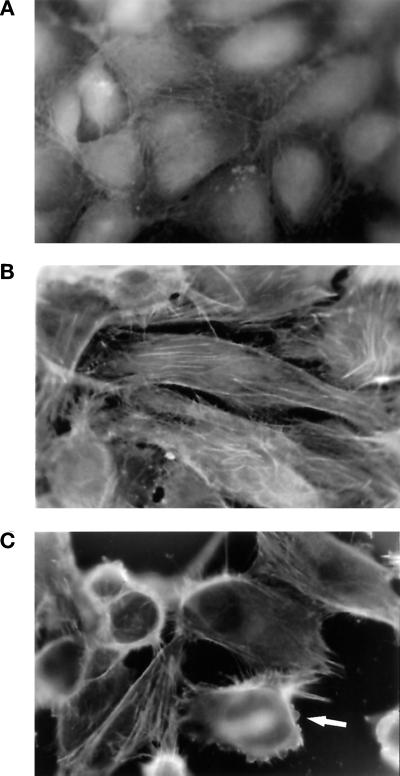
A time course analysis of thrombin-induced reorganization of the actin cytoskeleton in endothelial cells is shown in Figure 2. Two minutes after thrombin addition we commonly observe an increase in stress fiber formation. Staining with an anti-vinculin antibody revealed that the newly formed stress fibers terminate at focal adhesion complexes (our unpublished observations). After 5 min it is possible to detect some round cells, but cell rounding in response to thrombin is maximal after 15–30 min. Between 2 and 15 min, a pronounced ring of F-actin accumulates at the periphery of rounding cells (Figure 2C) and focal adhesion complexes disappear. In cells that do not round up, stress fibers thicken and become more organized. We have noted that the extent of cell retraction varies slightly from culture to culture and that more cells become totally round in EA.hy926 cultures than in primary HUVEC monolayers. Nonetheless, after treatment of both primary cultures and EA.hy926 cells with 10 nM thrombin, nearly all the cell–cell contacts become disrupted, and considerable gaps appear in the monolayer. As illustrated in Figure 2C, as cells round up they remain connected to one another by slender F-actin–containing retraction fibers. Maximum cell rounding is accompanied by the formation of blebs in the plasma membrane (Figures 1F and 2C). Bleb formation in these cells is not an indication of apoptosis, because no FITC-annexin-V staining could be detected in cells after thrombin treatment (our unpublished results). Rather, thrombin-induced reorganization of the actin cytoskeleton is completely reversible. Three hours after exposure to thrombin, confluent monolayers are reformed, consistent with the results of Rabiet et al. (1996).
Figure 2.
Thrombin-induced rounding of endothelial cells is preceded by stress fiber formation. Confluent EA.hy926 monolayers were stained with FITC-phalloidin after treatment with medium alone (A), 10 nM thrombin for 2 min (B) or 10 nM thrombin for 15 min (C). Photographs (magnification, 1000×) are representative of three different experiments. Arrow indicates cell surface blebbing.
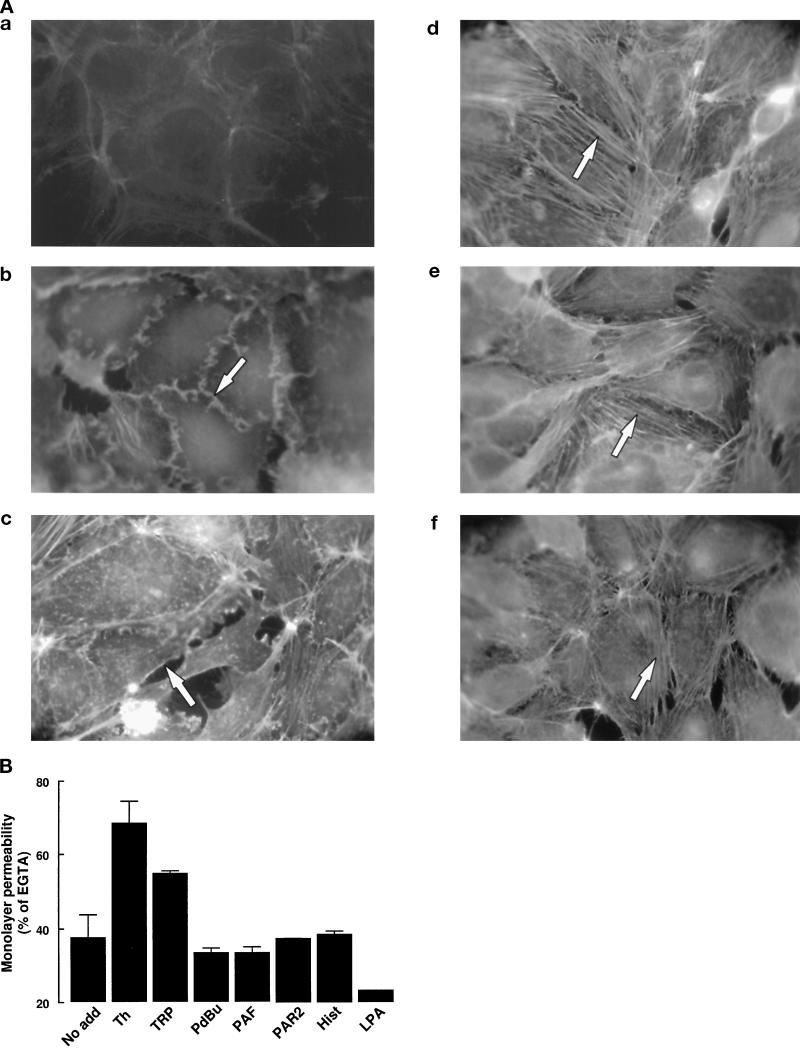
We next compared the thrombin-induced cytoskeletal effects on endothelial cells with the effects induced by other agonists, including agents known to increase endothelial permeability such as phorbol esters, PAF, and histamine (reviewed in Lum and Malik, 1994). As shown in Figure 3A, b, addition of 100 nM PdBu, a phorbol ester that activates most isoforms of protein kinase C, causes the appearance of edge ruffles around the entire perimeter of cells. In contrast to thrombin treatment, PdBu did not induce cell rounding or pronounced gap formation in the monolayer. PAF, a vasoactive lipid that is produced by endothelial cells in response to thrombin (Prescott et al., 1984), causes a rapid (≤5 min) and transient increase in stress fiber formation and peripheral F-actin staining in EA.hy926 cells (Figure 3A, c). The pattern of actin accumulation at the plasma membrane in PAF-treated cells is considerably less regular than in PdBu-treated cells, and we did note the appearance of small gaps in the monolayers. However PAF, like PdBu, does not mimic thrombin’s effect on cell rounding. This finding, together with the fact that pretreatment of cells with a PAF receptor antagonist (the WEB-2170 compound from Boehringer Ingelheim, Heidelberg, Germany) does not inhibit thrombin-induced cytoskeletal changes (our unpublished results), indicates that thrombin action cannot be accounted for by PAF release.
Figure 3.
Thrombin-induced rounding of endothelial cells is linked to an increase in monolayer permeability. (A) Medium alone (a), 100 nM PdBu (b), 1 nM PAF (c), 100 μM PAR-2 agonist peptide (d), 10−4 M histamine (e), or 20 ng/ml LPA (f) was added to confluent monolayers of EA.hy926 cells for 15 min (or 5 min in the case of PAF), and the actin cytoskeleton was visualized as described in MATERIALS AND METHODS. Arrows indicate membrane ruffling (b and c), and stress fibers (d–f). Photographs (magnification, 1000×) are representative of results from three independent experiments. (B) 10 nM thrombin, 100 μM TRP, 100 nM PdBu, 1 nM PAF, 100 μM PAR2, 10−4 M histamine, and 20 ng/ml LPA was added to confluent EA.hy926 cells grown on transwell filters, and the increase of transmonolayer permeability was determined after 15 min, as described in MATERIALS AND METHODS. Mean values ± SE obtained from four separate filters are represented.
In addition to the thrombin receptor PAR-1, endothelial cells have recently been shown to express a functional PAR-2 (Mirza et al., 1996; Nystedt et al., 1996; Molino et al., 1997). Indeed, we found that addition of 100 μM PAR-2 agonist peptide to EA.hy926 cells (Figure 3A, d) or to HUVECs induces the formation of stress fibers without inducing cell rounding. Histamine and LPA (Figure 3A, e and f) similarly increase the appearance of actin cables that extend across the entire cell. In contrast to thrombin, histamine and LPA do not markedly disrupt cell–cell contacts.
To evaluate the consequences of the observed agonist-induced cytoskeletal effects on endothelial barrier function, we assayed the passage of FITC-dextran through the monolayer after 15 min of treatment. As shown in Figure 3B, thrombin and the thrombin receptor agonist peptide (TRP) induced a significant increase in permeability to FITC-dextran, whereas PdBu, PAF, PAR-2 agonist, histamine, and LPA had little or no detectable effect. Thus, the ability of agonists to increase monolayer permeability in this in vitro assay correlates well with their ability to stimulate cell rounding. This system, however, may not fully reflect agonist-induced permeability changes observed in vivo, given the structural and cellular complexity of intact blood vessels.
Activation of Protein Kinase C Does Not Induce Endothelial Cell Rounding
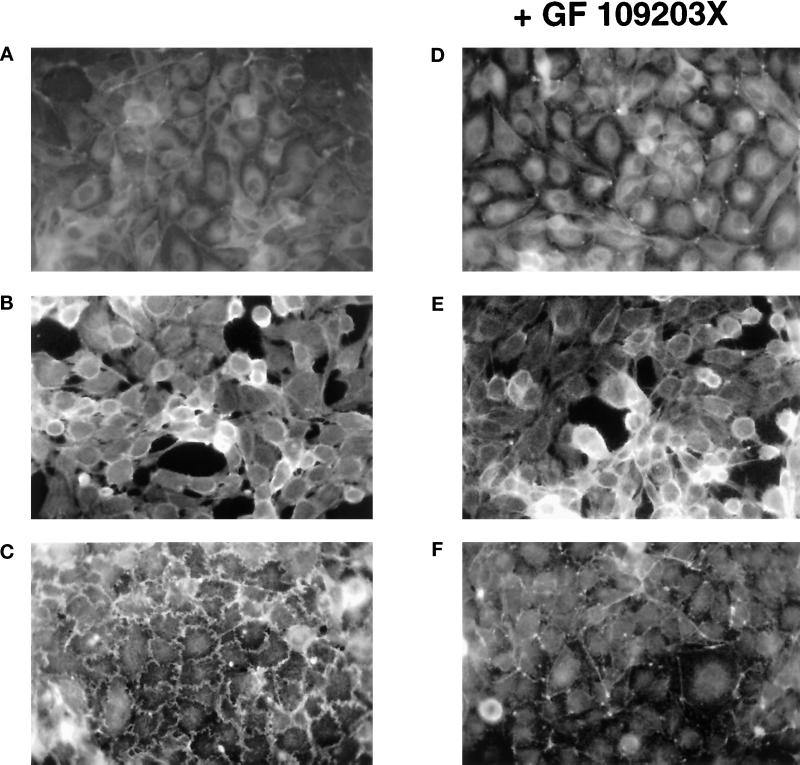
It has been well established that thrombin activates phospholipase C in target cells generating the second messengers diacylglycerol, an endogenous activator of protein kinase C, and inositol triphosphate, which induces release of Ca2+ from intracellular stores (for review, see Van Obberghen-Schilling and Pouysségur, 1993). A number of studies have suggested that a protein kinase C-dependent pathway is implicated in endothelial barrier function and actin reorganization in response to inflammatory mediators, including thrombin (reviewed in Lum, 1994). These studies have been based largely on the use of inhibitors such H-7, calphostin C, or staurosporine, which potently inhibit protein kinase C but also a variety of other protein kinases. We used the bisindoylmaleimide GF 109203X (Toullec et al., 1991), a more selective protein kinase C inhibitor that acts on the kinase domain (rather than the ATP binding site or the regulatory domain), to evaluate the contribution of protein kinase C in the cytoskeletal effects of thrombin. As shown in Figure 4, under conditions in which PdBu-induced ruffling is completely blocked, GF 109203X does not inhibit endothelial cell rounding induced by thrombin. Furthermore, overnight treatment of cells with PdBu to desensitize phorbol ester–sensitive isoforms of protein kinase C does not attenuate the response of cells to thrombin (our unpublished results). Together, these results suggest that protein kinase C activation is not sufficient to stimulate the thrombin-induced endothelial cell rounding.
Figure 4.
PKC inhibitor GF 109203X has no effect on thrombin-induced endothelial cell rounding. Confluent monolayers of EA.hy926 cells were pretreated for 30 min with the PKC inhibitor GF 109203X (D–F), and then medium (A and D), 10 nM thrombin (B and E), or 100 nM PdBu (C and F) was added to the cells for 15 min. F-actin staining was then performed as described in MATERIALS AND METHODS. Representative results from two independent experiments are shown (magnification, 400×).
BAPTA-AM was used to address the role of Ca2+i in thrombin-stimulated cytoskeletal reorganization. Pretreatment of cells for 45 min with 10 μM BAPTA-AM does not alter the thrombin effects (Table 1), whereas it abolishes the rapid increase of Ca2+i induced by thrombin (our unpublished results). Increasing concentrations of BAPTA-AM (up to 100 μM) only slightly reduce thrombin-stimulated cell retraction but do not inhibit peripheral cell actin accumulation. Similarly, artificially elevating Ca2+i with thapsigargin was without effect on cell morphology and did not inhibit the response to thrombin (our unpublished results). Thus, we conclude from these experiments that the phospholipase C–protein kinase C pathway is not required for the rounding of EA.hy926 cells or primary cultured HUVECs.
Table 1.
Effects of signaling pathway inhibitors on thrombin-induced endothelial cell rounding
| Inhibitor | Concentration | Signaling pathways | Cell rounding retraction |
|---|---|---|---|
| None | +++ | ||
| Pertussis toxin | 10 ng/ml | Gi/o | +++ |
| 8-bromo-cAMP | 1 mM | PKA (activation) | +++ |
| Wortmannin | 100 nM | PI-3 kinase | +++ |
| LY294002 | 5 μg/ml | ||
| Rapamycin | 4 μg/ml | S6 kinase | +++ |
| PD98059 | 30 μM | MAPK/ERK kinase | +++ |
| (P42/44 MAP kinase) | |||
| SB203580 | 5 μM | P38 MAP kinase | +++ |
| BAPTA-AM | 10 μM | CA2+ | ++ |
| Genistein | 100 μM | Tyrosine kinases | ++ |
| PP1 | 10 nM | Src family kinases | +++ |
| Staurosporine | 50 nM | Protein kinases (PKC, Src-like, Rho kinase,...) | − |
| KT5926 | 1 μM | Myosin light chain kinase | − |
Involvement of Additional Signaling Pathways in Thrombin-induced Rounding of Endothelial Cells
As summarized in Table 1, we examined the contribution of other known thrombin-stimulated signaling pathways in actin reorganization by the protease. The proliferative effects of thrombin are mediated, at least in part, by a pertussis toxin–sensitive heterotrimeric G protein (Chambard et al., 1987). Inhibition of this pathway with pertussis toxin had no effect on F-actin staining in control or thrombin-stimulated cells. Elevation of cAMP with 8-bromo-cAMP, under conditions in which protein kinase A activity is maximally increased, did not prevent thrombin-induced stress fiber formation or cell rounding in EA.hy926 cultures. Similarly, the thrombin response was not altered after pretreatment with forskolin and 1-methyl-3-isobutylxanthine (our unpublished results). However, we did note that primary HUVECs are more sensitive than EA.hy926 cells to inhibitory effects of cAMP-elevating agents, consistent with results previously reported in studies on thrombin-induced endothelial barrier dysfunction (Patterson et al., 1994).
Several lines of evidence suggest a link between phosphoinositide metabolism and regulation of the actin cytoskeleton. However, the PI 3-kinase pathway does not appear to mediate cytoskeletal remodeling by thrombin, because two unrelated inhibitors of PI 3-kinase activity, wortmannin and LY 294002, failed to block the actin rearrangement observed in thrombin-treated cells. Furthermore, S6 kinase inhibition with rapamycin had no apparent effect on the response of cells to thrombin. We also assessed the contribution of both the p42/44 MAP kinase and the p38 MAP kinase pathways by using the inhibitors PD98059 (an inhibitor of MAPK/ERK kinase) and SB203580 (an inhibitor of the p38 MAP kinase). The lack of effect of these compounds on thrombin-induced stress fiber formation and rounding suggests that these MAP kinase modules are not required.
It was recently demonstrated that at least one tyrosine kinase is involved in LPA-induced formation of stress fibers (Ridley and Hall, 1994; Nobes et al., 1995). To determine the possible involvement of tyrosine kinase(s) in thrombin-stimulated shape changes in endothelial cells, we examined the effect of genistein. As shown in Table 1, pretreatment of the cells with genistein decreases thrombin-induced cell rounding. However, tyrosine kinases of the Src family do not appear to be involved, because a recently described Src family kinase inhibitor, PP1 (Hanke et al., 1996), had no effect alone on the actin cytoskeleton of endothelial cells and did not detectably modify the effect of thrombin.
In contrast, the broad-spectrum protein kinase inhibitor staurosporine potently blocked thrombin-induced cell rounding. Interestingly, it did not inhibit stress fiber formation by thrombin (Figure 5E). Rather, thrombin-induced stress fibers appeared to be denser and longer in the presence of staurosporine. Because a comparable phenotype was observed with KT5926 (Figure 5F), it is possible that myosin light chain kinase is the major target of staurosporine for this effect. It is of interest to note that we obtained the same results when the thrombin receptor peptide was used to stimulate cells rather than thrombin. Furthermore, primary HUVEC cultures behaved similarly (our unpublished results).
Figure 5.
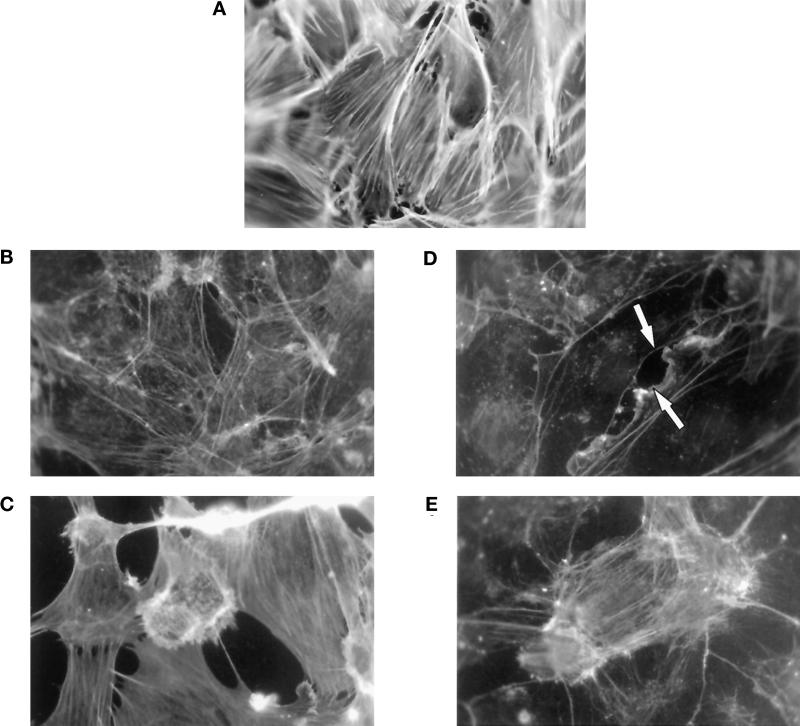
Staurosporine and KT5926 pretreatment prevent the rounding of endothelial cells. Confluent EA.hy926 cells were pretreated for 30 min with 50 nM staurosporine (B and E), 1 μM KT5926 (C and F), or DMSO alone (A) before the addition of 10 nM thrombin (D–F) for an additional 15 min. Staining of the actin cytoskeleton was then performed as described in MATERIALS AND METHODS. Photographs (magnification, 1000×) are representative of four independent experiments.
Rho-dependent Stress Fiber Formation Favors Monolayer Integrity
Increasing evidence indicates that regulation of the actin cytoskeleton involves small GTPases of the Rho family. It has been clearly demonstrated that Rho A activation controls the formation of stress fibers in fibroblasts (Ridley et al., 1992). In neuronal cells, thrombin-induced retraction of neurites has been found to require Rho A (Jalink et al., 1994). To investigate the role of Rho in the phenotypic changes induced by thrombin in HUVECs, we used Rho-modifying bacterial toxins. First we examined the consequences on the endothelial cell cytoskeleton of constitutive Rho activation with Escherichia coli CNF1 (Flatau et al., 1997). As shown in Figure 6A, an overnight treatment of cells with 10−9 M CNF1 induces the formation of thick transversal stress fibers. Rho activation does not lead to cell rounding; rather, CNF 1-treated cells acquire a flattened appearance. This effect correlates well with a decrease in agonist-stimulated permeability to macromolecules observed in CNF1-treated HUVECs (our unpublished results), thus providing a link between increased stress fiber formation and maintenance of endothelial permeability and suggesting that stress fibers contribute to barrier-promoting tethering forces.
Figure 6.
Implication of Rho in control of the endothelial cell actin cytoskeleton. Confluent HUVEC monolayers were pretreated with 10−9 M CNF1 for 16 h (A) or 10−7 M DC3B for 50 h (D and E). Thrombin (10 nM) was added during the last 15 min of the experiment in C and E. The actin cytoskeleton was stained with FITC-phalloidin as described in MATERIALS AND METHODS. Photos (magnification, 1000×) are representative of at least four independent experiments. Arrows indicate gap formation between bordering cells.
Alternatively, Rho function was inhibited by incubating cells with a chimeric toxin, DC3B, composed of Clostridium botulinum C3 exoenzyme and the B fragment of diphtheria toxin (Aullo et al., 1993). As shown in Figure 6D, preincubation of confluent HUVEC monolayers with 10−7 M DC3B for 50 h leads to the disappearance of longitudinal stress fibers and cytoplasmic F-actin staining. Staining became restricted to the periphery of cells and revealed a modest disruption of cell–cell contacts. Under these conditions of toxin treatment ∼75% of endogenous Rho is ADP ribosylated, as determined by an in vitro assay (our unpublished results). When DC3B-pretreated cells were stimulated with 10 nM thrombin for 15 min (Figure 6E), we observed an attenuation of cell rounding, compared with control thrombin-stimulated cells (Figure 6C). Similarly, partial inhibition of cell retraction by the toxin was observed in EA.hy926 cells. In the cell line it was clear, however, that inhibition of Rho did not inhibit actin polymerization beneath the plasma membrane, indicating that Rho may not be required for this effect. It is noteworthy that treatment of endothelial cells with the intact B fragment of diphtheria toxin, CRM197, had no effect on the cytoskeletal morphology of nontreated or thrombin-treated monolayers (our unpublished results).
A Rac-dependent Pathway Is Implicated in Thrombin-stimulated Cell Rounding
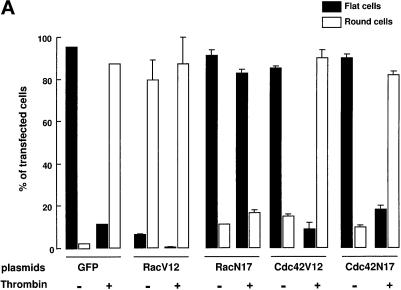
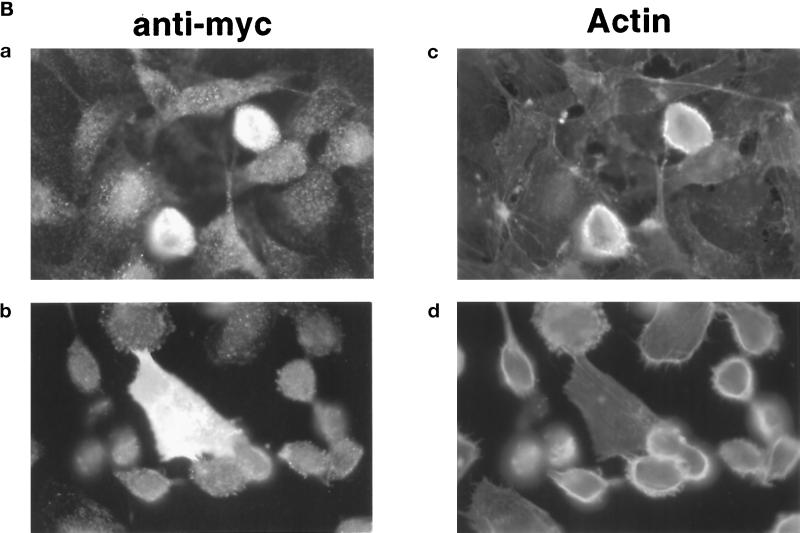
We next examined the involvement of Rac and Cdc42 GTPases in thrombin-induced cytoskeletal modifications. To do so, EA.hy926 cells near confluence were transiently transfected with plasmids encoding constitutively active or dominant-interfering mutants, and their effect on the actin cytoskeleton was analyzed. Examination of control cultures expressing green fluorescent protein indicated that ∼20% of the cells expressed the transfected construct, and the transfection conditions per se were without effect on cell morphology. The results obtained after transfection with the GTPase mutants, summarized in Figure 7A, reveal that Rac, and not Cdc42, is implicated in cell rounding by thrombin. Indeed, the expression of a constitutively active form of Rac (RacV12) led to the accumulation of cortical actin and cell rounding, in the absence of thrombin (Figure 7B, a and c). The addition of thrombin only slightly increased the number of round positive cells. A striking inhibition of thrombin-induced cell rounding and retraction was observed in cells transfected with the plasmid encoding pEXVRacN17, as shown in Figure 7B, b and d, strongly suggesting that Rac participates in the signaling pathway(s) that lead to this effect. In contrast, expression of constitutively active and dominant-interfering forms of Cdc42 had no functional consequences on either resting EA.hy926 cell morphology or thrombin-induced cytoskeletal modifications.
Figure 7.
Implication of Rac and Cdc42 in thrombin-induced cell rounding. (A) EA.hy926 cells were transfected with plasmids encoding green fluorescent protein (GFP) or myc-tagged GTPase mutants, as described in MATERIALS AND METHODS. The morphology of transfected cells, treated or not for 15 min with 10 nM thrombin, was observed, and 100 positive cells were scored (flat or round) per transfection condition. Data represent the mean of two independent experiments. (B) EA.hy926 cells were transfected with pEXVRac1V12 (a and c) or pEXVRac1N17 (b and d). Thrombin was added (10 nM) to cells shown in B and D 15 min before staining. Expression of Rac mutants was detected using anti-Myc antibody, and F-actin was stained with FITC-phalloidin.
DISCUSSION
We have examined the signal-transducing systems that mediate reorganization of the actin cytoskeleton in cultured HUVECs treated with thrombin. The present findings extend earlier studies documenting the effects of thrombin and other inflammatory mediators on endothelial permeability in vitro and barrier function in vivo. Increased permeability in endothelial monolayers results from both disruption of tethering forces, arising from cell–cell junctions and cell–matrix interactions, and enhancement of cell retraction (reviewed in Lum and Malik, 1994; Garcia et al., 1996). The actin cytoskeleton plays a prominent role in coordinating these events. Indeed, cell–cell adherens junctions are anchored to the actin cytoskeleton via a complex network of signaling molecules and cytoskeletal proteins. The cytoplasmic tail of integrins attaches to actin stress fibers at dynamic structures called focal adhesions. Finally, contraction directed toward the cell center requires actin binding to its functional partner myosin. Thus, understanding the molecular events underlying control of the cytoskeleton is essential for understanding regulation of endothelial permeability in response to thrombin.
The effect of thrombin is extensive and reversible and mediated by the G protein-coupled thrombin receptor PAR-1, which becomes rapidly desensitized after activation (Woolkalis et al., 1995). Recently, a second protease-activated thrombin receptor designated PAR-3 has been cloned (Ishihara et al., 1997). We have been able to detect very low levels of PAR-3 mRNA in EA.hy926 cells and HUVECs by Northern analysis. Nonetheless, PAR-1 mRNA expression is significantly higher (our unpublished results). In contrast to PAR-1, synthetic peptides corresponding to the amino acid sequence adjacent to the proposed thrombin cleavage site of PAR-3 are unable to activate the receptor (Ishihara et al., 1997). Therefore, it is impossible at present to determine the precise contribution of PAR-3 in thrombin’s effect on HUVECs. However, the fact that the PAR-1 agonist peptide is a full agonist of thrombin action strongly suggests that the contribution of PAR-3 to thrombin-stimulated cytoskeletal reorganization in human endothelial cells is minimal.
Our finding that protein kinase C is not an absolute requirement for thrombin-induced cytoskeletal rearrangement in endothelial cells is based on the use of GF 109203X under conditions that completely block phorbol ester-induced cell ruffling. This result is in apparent contradiction with results from other studies addressing endothelial barrier dysfunction (Lynch et al., 1990; Lum and Malik, 1994; Garcia et al., 1996; Rabiet et al., 1996; Aschner et al., 1997). However, similar to other studies we observe inhibition of thrombin’s effect using nonselective protein kinase C inhibitors such as calphostin C (our unpublished results). Because calphostin C acts on the diacyl glycerol-binding domain of protein kinase C, it is conceivable that a protein containing diacyl glycerol-binding zinc fingers, other than protein kinase C family members, may be involved in this effect.
Altogether, our results allow us to propose a model for thrombin-stimulated endothelial cell rounding, which involves multiple signaling pathways, including 1) a Rho-dependent pathway that controls stress fiber formation and cell spreading, 2) a Rac-stimulated pathway that regulates cortical actin polymerization, and 3) a myosin light chain kinase-dependent mechanism involved in cell retraction, as previously described (Garcia and Schaphorst, 1995). Whereas Rho appears to favor barrier function, the latter two events contribute to barrier dysfunction.
It has been clearly demonstrated in serum-starved 3T3 fibroblasts that formation of stress fibers and focal adhesion complexes are dependent on the activation of Rho (Ridley and Hall, 1992). More recently, results obtained in several laboratories have led to a converging hypothesis associating Rho to bundling of actin filaments via the activation of Rho kinase. It has been demonstrated that Rho kinase can phosphorylate the myosin-binding subunit of myosin phosphatase, thereby inactivating it (Kimura et al., 1996). In addition, Rho kinase has been shown to phosphorylate myosin light chain directly and to activate the ATPase (Amano et al., 1996). Both events lead to an increase in the phosphorylation state of myosin light chain and enhance binding of myosin to actin filaments (Chrzanowska-Wodnicka and Burridge, 1996). Accordingly, in resting endothelial cells, myosin ATPase inhibition with butanediome monoxime results in disappearance of stress fibers, disorganization of cell–cell contacts, and loss of cell adhesion to the substratum (unpublished observations).
We propose that the Rho-dependent pathway is associated with the maintenance of endothelial barrier function. This proposal is based on two observations. First, agonists that increase stress fiber formation in endothelial cells, such as LPA, PAR-2 peptide agonist, and histamine, do not increase the monolayer permeability to FITC-dextran. Second, constitutive activation of Rho by CNF1 induces prominent stress fiber formation without cell rounding, whereas inhibition of Rho by DC3B is associated with a loss of stress fibers and decreased cell adhesion. Accordingly, in primary cultured HUVECs, monolayer permeability was not increased in response to CNF1, whereas it was slightly augmented in response to DC3B. Our finding that C3 exoenzyme attenuates thrombin-stimulated endothelial cell retraction in HUVEC monolayers, without completely inhibiting it, is difficult to interpret. We verified that a significant proportion of Rho becomes ADP ribosylated in toxin-treated cells, compared with other systems (Fujihara et al., 1997). It suggests that Rho may influence the outcome of other signaling pathways. It is noteworthy that Rho mediates thrombin-stimulated shape changes in different cell types. However, the nature of the changes is cell type specific. For example, in neuronal cells, Jalink et al. (1994) have shown that thrombin promotes neurite retraction and cell rounding, whereas in astrocytes thrombin induces cell spreading (Suidan et al., 1997).
Myosin light chain phosphorylation is critical to thrombin-mediated endothelial cell contraction, as seen here by inhibition with KT5926 (see Figure 5; Wysolmerski and Lagunoff, 1990; Garcia et al., 1995). The fact that, in our hands, BAPTA-AM only modestly inhibits thrombin-induced endothelial cell retraction suggests that the myosin light chain kinase(s) implicated in this response is not strictly Ca2+/calmodulin dependent. Recently, a nonmuscle myosin light chain kinase has recently been cloned in endothelial cells (Garcia et al., 1997); however, signaling pathways involved in regulating this kinase remain to be elucidated.
Finally, we propose that thrombin-induced cortical actin accumulation is necessary for cell retraction. A growing number of studies confirm a role for Cdc42 and Rac in mediating signals from extracellular agonists to actin polymerization and remodeling beneath the plasma membrane (for recent review, see Lim et al., 1996; Tapon and Hall, 1997). For example, Cdc42 has been implicated in the formation of peripheral actin-containing structures including filopodia (Kozma et al., 1996) and Rac in the formation of membrane ruffles by platelet-derived growth factor (Ridley et al., 1992). Rac has also been reported to promote actin polymerization after thrombin treatment of human platelets through uncapping of existing filament ends (Hartwig et al., 1995). Here we demonstrate that Rac participates in cortical actin polymerization in endothelial cells challenged with thrombin. p21-activated kinases and PI kinases have been described as functional partners of this GTPase and both have been reported to be stimulated by thrombin (reviewed in Lim et al., 1996). We are currently examining the role of p21-activated kinases in thrombin-induced actin remodeling in endothelial cells.
In conclusion, the studies presented here highlight the complexity of the signaling systems that modulate changes in the cell cytoskeleton of endothelial cells challenged with thrombin. In light of the finding that agents that block thrombin-induced cell retraction increase stress fiber formation in the presence of thrombin, we propose that thrombin first activates a Rho-dependent pathway, which regulates barrier function, and then a Rac-dependent pathway involved in cell rounding and barrier dysfunction. In other systems, both interdependence and antagonism have been observed between Rho, Cdc42, and Rac pathways, reflecting the complexity of their regulation (see Lim et al., 1996; Kozma et al., 1997). Defining the molecular links between the thrombin receptor and Rho family GTPases as well as effector proteins that execute their effects remains a future challenge.
ACKNOWLEDGMENTS
We thank D. Grall for excellent technical assistance and P. Preau and Y. Fantei for the computer graphics. K.C. Malcolm is gratefully acknowledged for helpful discussions and critical reading of the manuscript. These studies were supported by the Centre National de la Recherche Scientifique and the University of Nice-Sophia Antipolis (UMR 6543), the Association pour la Recherche contre le Cancer, and the Ministère de l’Education Nationale de la Recherche et de la Technologie (grant ACC-SV 09). V.V.C. was supported by a research fellowship from the Sanofi Association for Thrombosis Research.
REFERENCES
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho- kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Aschner JL, Lum H, Fletcher PW, Malik AB. Bradykinin- and thrombin-induced increases in endothelial permeability occur independently of phospholipase C but require protein kinase C activation. J Cell Physiol. 1997;173:387–396. doi: 10.1002/(SICI)1097-4652(199712)173:3<387::AID-JCP11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Aullo P, Giry M, Olsnes S, Popoff MR, Kocks C, Boquet P. A chimeric toxin to study the role of the 21 kDa GTP binding protein rho in the control of actin microfilament assembly. EMBO J. 1993;12:921–931. doi: 10.1002/j.1460-2075.1993.tb05733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri B, Balconi G, Dejana B, Donati MB. Evidence that vascular endothelial cells can induce the retraction of fibrin clot. Proc Soc Exp Biol Med. 1981;168:204–207. doi: 10.3181/00379727-168-41260. [DOI] [PubMed] [Google Scholar]
- Chambard JC, Paris S, L’Allemain G, Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987;326:800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Chen Y-C, Grall D, Salcini E, Pelicci PG, Pouysségur J, Van Obberghen-Schilling E. Shc adaptor proteins are key transducers of mitogenic signaling mediated by the G protein-coupled thrombin receptor. EMBO J. 1996;15:1037–1044. [PMC free article] [PubMed] [Google Scholar]
- Chong LD, Traynor KA, Bokoch GM, Schwartz MA. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzano L, Fiorentini C, Donelli G, Michel E, Kocks C, Cossart P, Cabanie L, Oswald E, Boquet P. Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol Microbiol. 1993;9:1247–1254. doi: 10.1111/j.1365-2958.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Martin GS. Platelet tyrosine-specific protein phosphorylation is regulated by thrombin. Mol Cell Biol. 1988;8:3603–3610. doi: 10.1128/mcb.8.9.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Florentini C, Boquet P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- Fujihara H, Walker LA, Gong MC, Lemichez E, Boquet P, Somlyo AV, Somlyo AP. Inhibition of RhoA translocation and calcium sensitization by in vivo ADP-ribosylation with the chimeric toxin DC3B. Mol Biol Cell. 1997;8:2437–2447. doi: 10.1091/mbc.8.12.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol. 1995;163:510–522. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Lazar V, Gilbert ML, Gallagher PJ, Verin AD. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol. 1997;16:489–494. doi: 10.1165/ajrcmb.16.5.9160829. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Schaphorst KL. Regulation of endothelial cell gap formation and paracellular permeability. J Invest Med. 1995;43:117–126. [PubMed] [Google Scholar]
- Garcia JG, Verin AD, Schaphorst KL. Regulation of thrombin-mediated endothelial cell contraction and permeability. Semin Thromb Hemostasis. 1996;22:309–315. doi: 10.1055/s-2007-999025. [DOI] [PubMed] [Google Scholar]
- Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol. 1995;130:613–627. doi: 10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A, Brugge JS. Thrombin treatment induces rapid changes in tyrosine phosphorylation in platelets. Proc Natl Acad Sci USA. 1989;86:901–905. doi: 10.1073/pnas.86.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand RJ, Turnell AS, Grabham PW. Cellular consequences of thrombin-receptor activation. Biochem J. 1996;313:353–368. doi: 10.1042/bj3130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Hartwig JH, Bokoch GM, Carpenter CL, Janmey PA, Taylor LA, Toker A, Stossel TP. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Hong SL, Deykin D. Activation of phospholipases A2 and C in pig aortic endothelial cells synthesizing prostacyclin. J Biol Chem. 1982;257:7151–7154. [PubMed] [Google Scholar]
- Ishihara H, Connolly AJ, Zeng D, Kahn ML, Zheng YW, Timmons C, Tram T, Coughlin SR. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- Jalink K, van Corven E, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Tahamont MV, Malik AB. Thrombin-induced lung vascular injury. Roles of fibrinogen and fibrinolysis. Am Rev Respir Dis. 1983;128:38–44. doi: 10.1164/arrd.1983.128.1.38. [DOI] [PubMed] [Google Scholar]
- Kimura K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The GTPase-activating protein n-chimaerin cooperates with Rac1 and Cdc42Hs to induce the formation of lamellipodia and filopodia. Mol Cell Biol. 1996;16:5069–5080. doi: 10.1128/mcb.16.9.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laposata M, Dovnarsky DK, Shin HS. Thrombin-induced gap formation in confluent endothelial cell monolayers in vitro. Blood. 1983;62:549–556. [PubMed] [Google Scholar]
- Lim L, Manser E, Leung T, Hall C. Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras- related Rho subfamily and its role in phosphorylation signalling pathways. Eur J Biochem. 1996;242:171–185. doi: 10.1111/j.1432-1033.1996.0171r.x. [DOI] [PubMed] [Google Scholar]
- Lo SK, Perlman MB, Niehaus GD, Malik AB. Thrombin-induced alterations in lung fluid balance in awake sheep. J Appl Physiol. 1985;58:1421–1427. doi: 10.1152/jappl.1985.58.5.1421. [DOI] [PubMed] [Google Scholar]
- Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol. 1994;267:L223–L241. doi: 10.1152/ajplung.1994.267.3.L223. [DOI] [PubMed] [Google Scholar]
- Lynch JJ, Ferro TJ, Blumenstock FA, Brockenauer AM, Malik AB. Increased endothelial albumin permeability mediated by protein kinase C activation. J Clin Invest. 1990;85:1991–1998. doi: 10.1172/JCI114663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza H, Yatsula V, Bahou WF. The proteinase activated receptor-2 (PAR-2) mediates mitogenic responses in human vascular endothelial cells. J Clin Invest. 1996;97:1705–1714. doi: 10.1172/JCI118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molino M, Woolkalis MJ, Reavey CJ, Pratico D, Andrade GP, Barnathan ES, Brass LF. Endothelial cell thrombin receptors and PAR-2. Two protease-activated receptors located in a single cellular environment. J Biol Chem. 1997;272:11133–11141. doi: 10.1074/jbc.272.17.11133. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- Nystedt S, Ramakrishnan V, Sundelin J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J Biol Chem. 1996;271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- Patterson CE, Davis HW, Schaphorst KL, Garcia JG. Mechanisms of cholera toxin prevention of thrombin- and PMA-induced endothelial cell barrier dysfunction. Microvasc Res. 1994;48:212–235. doi: 10.1006/mvre.1994.1050. [DOI] [PubMed] [Google Scholar]
- Phillips PG. Thrombin-induced alterations in endothelial cell cytoarchitectural and functional properties. Semin Thromb Hemostasis. 1994;20:417–425. doi: 10.1055/s-2007-1001930. [DOI] [PubMed] [Google Scholar]
- Prescott SM, Zimmerman GA, McIntyre TM. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci USA. 1984;81:3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiet MJ, Plantier JL, Rival Y, Genoux Y, Lampugnani MG, Dejana E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler Thromb Vasc Biol. 1996;16:488–496. doi: 10.1161/01.atv.16.3.488. [DOI] [PubMed] [Google Scholar]
- Rasmussen UB, Vouret-Craviari V, Jallat S, Schlesinger Y, Pagès G, Pavirani A, Lecocq J-P, Pouysségur J, Van Obberghen-Schilling E. cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 1991;288:123–128. doi: 10.1016/0014-5793(91)81017-3. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO J. 1994;13:2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Suidan HS, Nobes CD, Hall A, Monard D. Astrocyte spreading in response to thrombin and lysophosphatidic acid is dependent on the Rho GTPase. Glia. 1997;21:244–252. doi: 10.1002/(sici)1098-1136(199710)21:2<244::aid-glia7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Toullec D, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Van Corven EJ, Hordijk PL, Medema RH, Bos JL, Moolenaar WH. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc Natl Acad Sci USA. 1993;90:1257–1261. doi: 10.1073/pnas.90.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E, Pouysségur J. Signaling pathways of the thrombin receptor. Thromb Haemostasis. 1993;70:163–167. [PubMed] [Google Scholar]
- Van Obberghen-Schilling E, Vouret-Craviari V, Chen Y-H, Grall D, Chambard J-C, Pouysségur J. Thrombin and its receptor in growth control. Ann NY Acad Sci. 1995;766:431–441. doi: 10.1111/j.1749-6632.1995.tb26692.x. [DOI] [PubMed] [Google Scholar]
- Vu TKH, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Woolkalis MJ, DeMelfi TM, Blanchard N, Hoxie JA, Brass LF. Regulation of thrombin receptors on human umbilical vein endothelial cells. J Biol Chem. 1995;270:9868–9875. doi: 10.1074/jbc.270.17.9868. [DOI] [PubMed] [Google Scholar]
- Wysolmerski RB, Lagunoff D. Involvement of myosin light-chain kinase in endothelial cell retraction. Proc Natl Acad Sci USA. 1990;87:16–20. doi: 10.1073/pnas.87.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, King WG, Dillon S, Hall A, Feig L, Rittenhouse SE. Activation of platelet phosphyatidylinositide 3-kinase requires the small GTP-binding protein Rho. J Biol Chem. 1993;268:22251–22254. [PubMed] [Google Scholar]
- Zhang J, Zhang J, Benovic JL, Sugai M, Wetzker R, Gout I, Rittenhouse SE. Sequestration of a G-protein beta gamma subunit or ADP-ribosylation of Rho can inhibit thrombin-induced activation of platelet phosphoinositide 3-kinases. J Biol Chem. 1995;270:6589–6594. doi: 10.1074/jbc.270.12.6589. [DOI] [PubMed] [Google Scholar]