Resting Respiratory Tract Dendritic Cells Preferentially Stimulate T Helper Cell Type 2 (Th2) Responses and Require Obligatory Cytokine Signals for Induction of Th1 Immunity (original) (raw)
Abstract
Consistent with their role in host defense, mature dendritic cells (DCs) from central lymphoid organs preferentially prime for T helper cell type 1 (Th1)-polarized immunity. However, the “default” T helper response at mucosal surfaces demonstrates Th2 polarity, which is reflected in the cytokine profiles of activated T cells from mucosal lymph nodes. This study on rat respiratory tract DCs (RTDCs) provides an explanation for this paradox. We demonstrate that freshly isolated RTDCs are functionally immature as defined in vitro, being surface major histocompatibility complex (MHC) II lo, endocytosishi, and mixed lymphocyte reactionlo, and these cells produce mRNA encoding interleukin (IL)-10. After ovalbumin (OVA)-pulsing and adoptive transfer, freshly isolated RTDCs preferentially stimulated Th2-dependent OVA-specific immunoglobulin (Ig)G1 responses, and antigen-stimulated splenocytes from recipient animals produced IL-4 in vitro. However, preculture with granulocyte/macrophage colony stimulating factor increased their in vivo IgG priming capacity by 2–3 logs, inducing production of both Th1- and Th2-dependent IgG subclasses and high levels of IFN-γ by antigen-stimulated splenocytes. Associated phenotypic changes included upregulation of surface MHC II and B7 expression and IL-12 p35 mRNA, and downregulation of endocytosis, MHC II processing– associated genes, and IL-10 mRNA expression. Full expression of IL-12 p40 required additional signals, such as tumor necrosis factor α or CD40 ligand. These results suggest that the observed Th2 polarity of the resting mucosal immune system may be an inherent property of the resident DC population, and furthermore that mobilization of Th1 immunity relies absolutely on the provision of appropriate microenvironmental costimuli.
Keywords: dendritic cell, lung, function, T helper cell type 1, T helper cell type 2
Mucosal surfaces such as those of the lung represent sites of intense exposure to a wide variety of inert and infectious agents and thus provide uniquely challenging microenvironments for the maintenance of immunological homeostasis. At such sites, the T cell system is required to respond rapidly to infectious challenge via the initiation of sterilizing immunity, while at the same time avoiding large scale inflammatory responses to inert, nonreplicating antigens. Disruption of this homeostasis, as in hyperreactivity to inhaled allergens, is central in the pathogenesis of many respiratory disorders, including rhinitis and asthma.
Within mucosal environments, APCs play a key regulatory role via sampling and presenting antigen in a form recognizable by the immune system. Among APC populations present within the mucosae, dendritic cells (DCs)1 are unique in being able to efficiently stimulate primary immunity. Previous studies from our (1, 2) and other (3, 4) laboratories have identified DCs as the principal resident APC of the rat, mouse, and human lung and have shown that these cells form a contiguous network throughout the airway epithelium (5), placing them in an ideal position to sample and process inhaled antigens.
The ability to rapidly acquire large amounts of antigen (6, 7), in conjunction with expression of high levels of MHC and costimulatory molecules (8, 9), makes DCs potent APCs both in vitro (10–12) and in vivo (13, 14). However, it has been suggested that the functional activities of individual DCs are closely linked to their maturational status, i.e., resident DCs in peripheral tissues are proposed to be of the “immature” type and are specialized for antigen uptake as opposed to activation of T cells, whereas the reverse applies for mature DCs in secondary lymphoid organs (15–17). This functional dichotomy provides a theoretical mechanism for avoidance of chronic T cell–mediated tissue damage at sites (especially mucosal) of continuous antigen exposure, i.e., transmission of activating signals to the T cell system by DCs normally only occurs after their migration to, and functional maturation within, central lymphoid organs, especially the draining lymph nodes (18).
An important functional role for mature DCs is considered to be in the clearance of infectious agents (19) facilitated by the rapid induction of high-level IL-12 production by these cells (20–22) and leading to upregulation of IFN-γ production and Th1-mediated responses (23). The mucosal surfaces of the gastrointestinal and respiratory tracts are the sites of most frequent contact with pathogens, and the dense and highly dynamic DC populations at these sites (5, 24–26) are believed to play key roles in orchestrating these host-protective immune responses. However, the initial response to inhaled soluble protein antigens is dominated by Th2-dependent IgE production (27–29) and/or the production of Th2 cytokines in the regional lymph nodes draining the airway mucosa (29, 30), and a comparable Th2 “default” is operative at the level of the gastric mucosa (31– 33). Furthermore, T cells isolated from lymph nodes that drain mucosal sites have been shown to predominantly produce IL-4 upon activation in vitro (34). Given that DCs are the principal APCs responsible for initiation of immune reactivity to both infectious and inhaled/ingested agents, the inherent Th1-promoting activity of DCs described above is inconsistent with the observations that immune responses at mucosal sites are essentially Th2 dominated.
To address this paradox, we have analyzed the functional activity of RTDCs in the resting state and after exposure to cytokines previously demonstrated to induce functional maturation of DCs from other sources.
Materials and Methods
Animals.
Inbred PVG.RT7b (RT1cRT7b), WAG (RT1u), or BN (RT1n) strain rats were bred free of common rat pathogens in-house at the Institute for Child Health Research and housed under conventional conditions. 8-wk-old female animals were generally used except in the case of lung donors, in which case older (>13 wk) animals were used.
Media and Reagents.
The tissue culture medium used was RPMI 1640 supplemented with 2 mM l-glutamine, 5 × 10-5 M 2-ME, and antibiotics, as well as either 5% FCS (Trace Biosciences, Melbourne, Australia) or 1% normal rat serum. Cell isolation procedures were performed in ice-cold PBS supplemented with 0.2% BSA (PBS/BSA) or a solution of 11 mM d-glucose, 5.5 mM KCl, 137 mM NaCl, 25 mM Na2HPO4, and 5.5 mM NaH2PO4 × 2H2O (GKN) supplemented with 5% FCS (GKN/ FCS) or 0.2% BSA (GKN/BSA) as indicated. Mouse rGM-CSF (Biosource International, Camarillo, CA) was used at a final concentration of 10 ng/ml. FITC-conjugated dextran (FITC-DX), M r = 40 kD, was purchased from Molecular Probes (Eugene, OR), and Collagenase A was from Boehringer Mannheim (Mannheim, Germany). AMV reverse transcriptase and Taq DNA polymerase were from Promega (Madison, WI).
mAbs and Cell-staining Reagents.
Mouse mAbs to rat CD4 (W3/25), CD8 (OX8), CD11b/c (OX42), CD45 (OX1), RT1.Ac (OX27), RT1.B (OX6), Ig κ chain (OX12), and human C3b inactivator (OX21) were donated by Dr. Don Mason, MRC Cellular Immunology Unit, University of Oxford, Oxford, UK; WT.1 (LFA-1α) and 1A-29 (ICAM-1) were donated by Dr. M. Miyasaka, Osaka University, Osaka, Japan; and 3H5 (CD80) and 24F (CD86) were donated by Dr. H. Yagita, Juntendo University, Tokyo, Japan. Conjugates of OX6 IgG with FITC (OX6-FITC) or biotin (OX6b) were prepared in-house and in the case of the OX6b were used in conjunction with streptavidin-PE (Serotec, Kidlington, UK). A mouse CTLA4–human Ig Fc fusion protein (CTLA4-Ig) cross reactive with rat B7 was provided by Dr. M. Dallman, Imperial College, London, UK. Isotype controls were used as TCS (OX21) or as IgG1-FITC or IgG1-PE conjugates (Dako, Denmark). Binding of mouse mAbs was detected using a goat anti–mouse IgG-PE (GAM-PE) conjugate (Dako) and CTLA4-Ig using a goat anti–human IgG-PE conjugate (Immunotech, Marseilles, France). Cell samples were analyzed for surface fluorescence by flow cytometry using an Epics XL flow cytometer (Coulter Corp., Miami, FL) with forward- and side-angle light scatter gating combined with fluorescence channel gating to collect a minimum of 5 × 103 OX6+ events. Rabbit antipan-cytokeratin antibody for immunostaining of epithelial cells was obtained from Nycomed Amersham (Little Chalfont, Buckinghamshire, UK); immunoperoxidase staining of cytospin preparations of DCs was performed via standard methods.
Cell Preparations.
Collagenase A digests of perfused lung tissue were obtained from 10–13-wk-old rats using a previously described procedure (1). Digests of whole lung tissue were depleted of endogenous macrophages (mφ) by nylon wool elution in GKN/FCS and washed in GKN/BSA, and residual contaminating B cells were removed by labeling with the OX12 mAb followed by magnetic depletion using anti–mouse IgG-coated MACS beads (Miltenyi Biotec, Bergisch Gladbach, Germany). After this procedure, contaminating mφ were undetectable and B cells were <5%. The depleted cells were then washed and incubated with GAM-PE to label residual OX12+ B cells, followed by blocking with 10% normal mouse serum and incubation with OX6-FITC to label MHC class II+ cells. OX6+OX12− RTDCs were then purified by dual-parameter cell sorting (Epics Elite; Coulter Corp.) using chilled GKN plus 20% FCS as the collection medium. Splenic DCs were prepared by essentially the same procedure using Collagenase A digests of rat spleen.
An initial series of experiments were performed using 88–94% pure DC preparation containing up to 3% contaminating epithelial cells as defined by cytokeratin staining. The study was subsequently replicated with DC preparations that were re-sorted to achieve 98% purity, with identical results.
Analysis of Endocytosis by Flow Cytometry and Confocal Microscopy.
For quantitative analysis of RTDC endocytic activity, whole lung digests were depleted of mφ and B cells as described above and labeled with OX6-biotin/streptavidin-PE. 5 × 105 cells were then resuspended in RPMI/FCS containing 0.5 mg/ml FITC-DX for 90 min either at 37°C or on ice (0°C). The reaction was stopped by washing in ice-cold PBS/BSA and mean FL1 (FITC) fluorescence intensity of OX6+ cells was determined by flow cytometry. Results from these mixed-cell assays were further confirmed using purified RTDCs. For confocal microscopy analysis of single-cell uptake, purified RTDCs were incubated with FITC-DX as described above, washed, and then fixed in PBS containing 1% paraformaldehyde. 105 cells were then allowed to settle onto glass coverslips and fluorescence distribution was analyzed by confocal laser microscopy (Bio-Rad Labs., Hercules, CA). Results of single z-plane analyses are reported.
MLR.
Purified fresh or GM-CSF–exposed RTDCs were resuspended in RPMI/FCS and added at a final concentration of 1.25 × 104 cells/well of round-bottomed 96-well microtiter plates, then serially diluted twofold. Allogeneic responder T cells were purified from WAG-strain cervical lymph nodes by nylon wool elution and added at a final concentration of 1.25 × 105 cells/well. The cultures were then incubated for 72 h followed by an 18-h pulse with 0.5 μCi of [3H]thymidine. Results are expressed as mean CPM ± SEM for triplicate wells.
In Vivo DC Transfer Protocol.
The adoptive transfer protocol used was adapted from the protocol of De Becker et al. (35) with minor modifications. Purified DCs were resuspended to 106 cells/ml in RPMI/FCS and pulsed at 37°C with 1 mg/ml OVA (Sigma Chemical Co., St. Louis, MO) for 90 min for fresh RTDCs or overnight in the presence of 10 ng/ml rGM-CSF for RTDCs and spleen DCs. In some cases cells were pulsed with OVA for 90 min and washed before culture in GM-CSF. The cells were then washed in protein-free PBS and 105 viable cells were transferred intravenously into 8-wk-old female syngeneic recipients. After 5 d the animals were challenged with 10 μg/ml OVA in PBS intravenously and serum samples were prepared 15 d after OVA challenge. In cases where ex vivo spleen cell cultures were required, the cells were pulsed with OVA before transfer in the presence of 1% autologous normal rat serum instead of FCS to avoid presentation of bovine proteins during the in vitro cultures. Control animals received 1 ml of PBS intravenously on day 0 and 10 μg OVA intravenously on day 5.
OVA-specific IgG Subclass Analysis.
OVA-specific IgG subclasses were assayed by ELISA using microtiter plates coated overnight at 4°C with 10 μg/ml OVA in PBS. The plates were blocked with 1% BSA for 60 min at room temperature and then incubated with serum samples serially diluted in PBS for 2 h at room temperature. After washing, bound IgG subclasses were detected using biotin-conjugated rabbit anti–rat IgG1, IgG2a, and IgG2b (Amersham Pharmacia Biotech, Piscataway, NJ) incubated for 2 h at room temperature followed by an anti–biotin–horseradish peroxidase conjugate (Boehringer Mannheim, Mannheim, Germany) for 60 min at room temperature then horseradish peroxidase substrate (K-Blue™; Neogen, Lexington, KY). OD at 450 nm was assessed after 20 min. The concentration of each IgG subclass was determined by comparison with standard curves run in parallel, generated by coating plates with dilutions of purified rat IgG1, IgG2a, and IgG2b (Amersham Pharmacia Biotech) followed by the detection system described above, and the results were expressed as micrograms per milliliter of IgG. Serum IgE titers were assayed by the PCA method as previously described (36).
Spleen Cell Proliferation and Cytokine Protein Assays.
Single cell suspensions (including RBCs) of whole spleens from DC transfer recipients were prepared 15 d after OVA challenge and incubated in 96-well microtiter plates at 1.25 × 105 cells/well in 0.2-ml volumes at 37°C in RPMI/FCS containing 50 μg/ml OVA. After 72 h, cell proliferation was assessed by [3H]thymidine incorporation added for 18 h and results were expressed as mean CPM ± SEM of triplicate wells. At the 72 h time point, culture supernatants were taken for analysis of rat IFN-γ protein by ELISA (29) and IL-4 by B cell bioassay using upregulation of MHC class II as previously described (37).
Semiquantitative Reverse Transcriptase PCR.
Total RNA was prepared from 105 purified RTDCs by RNAzol B extraction (Biogenesis, Poole, UK) according to the manufacturer's instructions and mRNA was reverse transcribed to cDNA. Semiquantitative analysis of mRNA levels was then performed by PCR using cDNA serially diluted in reaction buffer from neat to 1:25 to ensure that PCR products were being analyzed under nonsaturating conditions. Amplification of β-actin mRNA was to ensure equivalent cDNA levels between samples; any experiments showing disparities in β-actin levels were discarded. The sequences of PCR primer pairs used are listed in Table 1, whereas primers specific for mouse IL-12 p35 that cross-reacted with rat (expected product size 308 bp) were purchased from Biosource International. To determine mRNA/β-actin ratios, PCR products were separated on 1.5% agarose gels and ratios of mRNA/β-actin band densities at 1:5 dilutions of cDNA determined by densitometry.
Table 1.
Rat PCR Primer Sequences
| Target mRNA | Primer sequence (5′–3′) | Product size | |
|---|---|---|---|
| Sense | Antisense | ||
| bp | |||
| RT1.B | cag tca cag aag gcg ttt atg | gat cgc agg cct tga atg atg | 279 |
| RT1.DMa | aac ata ggg ctc tcc gag | atg aaa cag acc agc gtg | 281 |
| RT1.DMb | gtc caa gta gcc caa acc | acc acg cag gtg tag atg | 233 |
| Ii | tga aga atg tta cca agt atg g | tgg tca ata ctt tag gtg gag | 252 |
| Calnexin | tgt att gat gtc tcg ggc | act cac act tag ggt tgg | 725 |
| IL-10 | cac tgc tat gtt gcc tgc tc | ttc atg gcc ttg tag aca cc | 463 |
| IL-12 p40 | tgg agt cat agg ctc tgg a | gat gaa gaa gct ggt gct g | 484 |
| β-actin | atg cca tcc tgc gtc tgg acc tgg | agc att tgc ggt gca cga tgg agg g | 607 |
Statistics.
Statistical comparisons of mean values were performed using the nonparametric Mann-Whitney test for unpaired samples with two-tailed P value using the InStat software package (GraphPad Software, San Diego, CA).
Results
Surface Phenotype Freshly Isolated and GM-CSF–matured RTDCs.
Surface marker expression on fresh and GM-CSF–matured RTDCs was analyzed using mφ- and B cell– depleted lung digests colabeled with mAbs to a range of rat lymphoid and myeloid cell surface antigens in conjunction with the anti–rat MHC class II mAb OX6. Approximately 10% of cells within freshly isolated lung digests were putative RTDCs as determined by positive labeling for OX6 and failure to express mφ or B cell markers (data not shown) and, as depicted in Table 2, a significant proportion expressed low levels of MHC class I and class II, CD4 and CD11a, and a small percentage expressed ICAM-1 and CD45, whereas the majority were negative for CD8, CD11b/c, and B7. However, after overnight exposure to 10 ng/ml GM-CSF, dramatic increases in expression of MHC class I and class II and B7 were observed, with moderate increases in CD45 and CD11a and CD11b/c, and decreases in CD4 and CD54 (Table 2).
Table 2.
Surface Phenotype of Fresh and GM-CSF–exposed RTDCs*
| Marker | mAb | Fresh | Plus GM-CSF | ||
|---|---|---|---|---|---|
| Percentage frequencies | MFI | Percentage frequencies | MFI | ||
| CD4 | W3/25 | 29.7 | 4.5 | 2.9 | 2.7 |
| CD8 | OX8 | 2.0 | 3.6 | 2.5 | 6.0 |
| CD11a | WT.1 | 13.9 | 3.7 | 19.4 | 7.2 |
| CD11b/c | OX42 | 3.3 | 3.1 | 10.8 | 6.8 |
| CD45 | OX1 | 5.9 | 3.3 | 8.0 | 3.0 |
| CD54 | 1A-29 | 8.9 | 6.3 | 4.1 | 9.9 |
| CD80/86 | CTLA-4 Ig | 0.8 | 2.2 | 54.8 | 38.6 |
| MHC class I | OX27 | 18.7 | 5.4 | 62.0 | 10.2 |
| MHC class II | OX6 | 100 | 11.5 | 100 | 55.1 |
GM-CSF Upregulates the Antigen-presenting Activity of RTDCs.
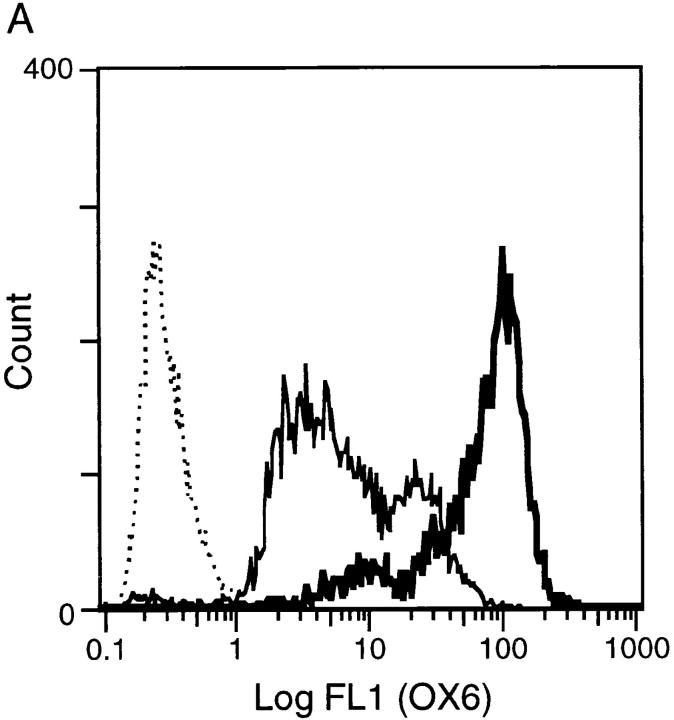
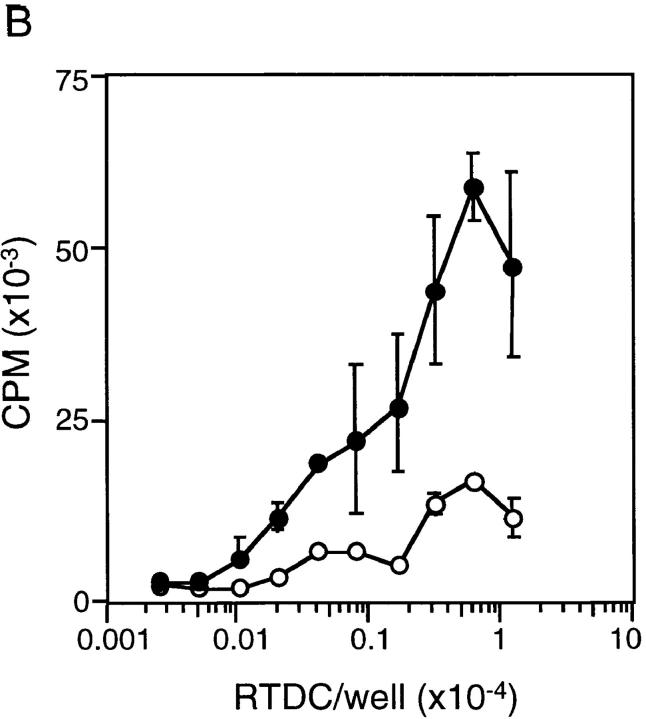
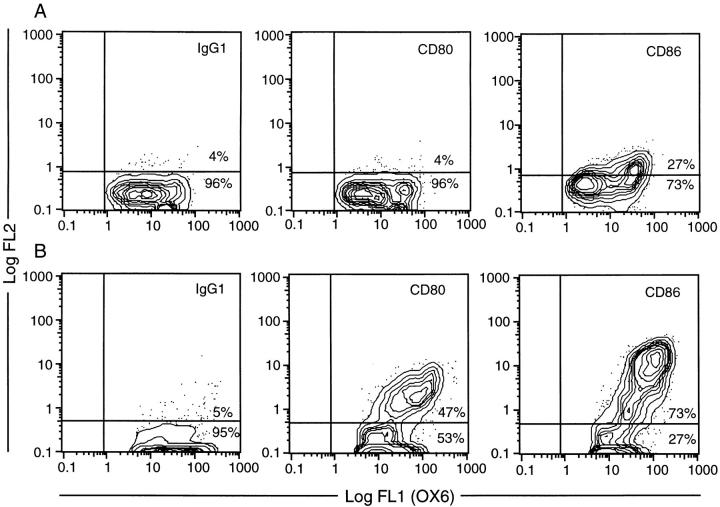
The phenotypic changes after exposure of freshly isolated RTDCs to GM-CSF described above, particularly the dramatic increases in MHC and B7 molecule expression, suggested that freshly isolated RTDCs may be functionally “immature” as APCs and that the observed maturational increase in surface expression of costimulatory molecules after exposure to GM-CSF should invoke a more potent APC activity. To investigate this, RTDCs were sorted to high purity on the basis of MHC class II expression as described in Materials and Methods and analyzed either as freshly isolated cells or after overnight exposure to GM-CSF. As shown in Fig. 1 A, exposure to 10 ng/ml GM-CSF resulted in an ∼10-fold increase in the level of surface MHC class II expression when compared with freshly isolated cells. In addition, this was associated with a marked increase in MLR-stimulating activity (Fig. 1 B), suggesting that RTDCs undergo functional maturation after exposure to GM-CSF. This was further confirmed by analysis of surface B7 expression, which revealed no expression of CD80 but low levels of CD86 on fresh RTDCs, the levels of which were both dramatically increased after exposure to GM-CSF, particularly on the MHC class IIhi subset (Fig. 2, A and B).
Figure 1.
Surface MHC class II expression and MLR-stimulating activity of fresh and GM-CSF–exposed RTDCs. (A) Purified RTDCs were labeled with OX6-FITC either as fresh cells (thin line) or after culture in GM-CSF (thick line), or with the isotype control IgG1-FITC (dotted line), and surface fluorescence analyzed by flow cytometry. (B) Serial dilutions of freshly purified (open circles) or GM-CSF–exposed (filled circles) RTDCs were added as stimulators to a primary allogeneic MLR in the presence of 105 purified WAG-strain T cells per well of 96-well microtiter plates. Cell proliferation (CPM) was assessed after a total of 96 h including an 18-h [3H]TdR pulse. Mean ± SEM of triplicate wells of one representative of three experiments is shown.
Figure 2.
CD80 and CD86 expression by fresh and GM-CSF–exposed RTDCs. Fresh (A) or GM-CSF–exposed (B) RTDC were colabeled with the OX21 (IgG1), 3H5 (CD80), or 24F (CD86) mAbs plus GAM-PE followed by OX6-FITC, and surface fluorescence analyzed by flow cytometry. Quadrant markers were set for IgG1-FITC/IgG1 plus GAM-PE negative control cells.
Modulation of the Endocytic Activity of RTDCs by GM-CSF.
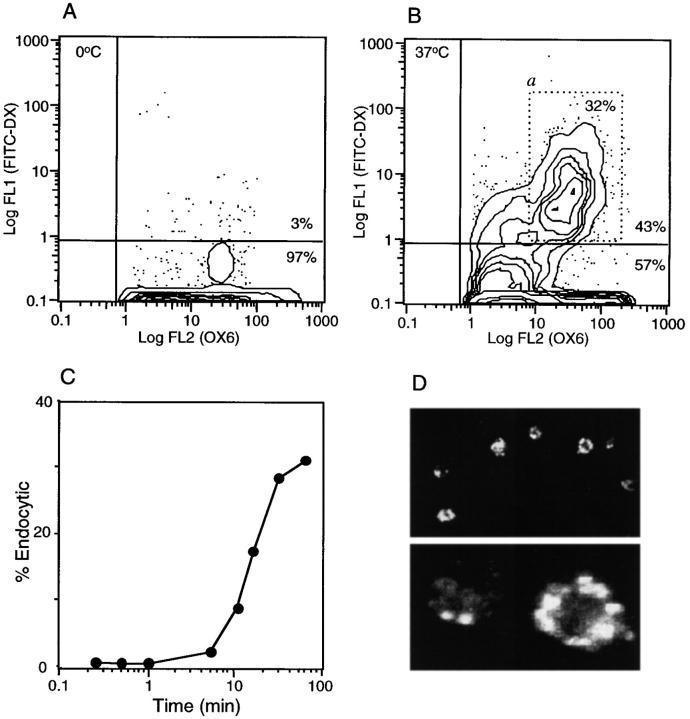
Recent studies of human peripheral blood–derived DCs have established that immature DCs are capable of high levels of receptor-mediated and fluid phase endocytosis, the levels of which are rapidly downregulated upon in vitro maturation (38). These changes are thought to occur in vivo when peripheral tissue DCs, specialized for antigen acquisition, mature and migrate to draining lymph nodes, where their primary role becomes T cell activation. To analyze the endocytic activity of RTDCs, the uptake of FITC-DX by freshly isolated and GM-CSF matured RTDCs was assessed. Freshly isolated RTDCs were actively endocytic, as indicated by their ability to acquire high levels of FITC-DX at 37°C (Fig. 3, A and B). The majority of this endocytic activity was confined to an MHC class IIint subset, which represented ∼30% of the total RTDC population (Fig. 3 B, a), although a distinct MHC class IIhi population that was negative for endocytosis was also present. This latter population may represent a more mature subset of cells that have downregulated endocytic activity (see below). Time course analysis of the uptake of FITC-DX by this subset showed detectable endocytic activity after 5 min with a peak at 60 min (Fig. 3 C).
Figure 3.
Endocytic activity of fresh RTDC. Freshly purified RTDC were incubated at 0°C (A) or 37°C (B) for 90 min in the presence of 0.5 mg/ml FITC-DX then labeled with the OX6 mAb plus GAM-PE and fluorescence levels analyzed by flow cytometry after trypan blue quenching. (C) Time-course of FITC-DX uptake by fresh RTDCs. RTDCs were incubated with FITC-DX as described above and the percentage of endocytically positive cells falling within an arbitrary gating region (B, a) determined by flow cytometry. Percentage endocytic values were calculated by subtracting values obtained at 0°C from those obtained at 37°C. One representative out of three experiments is shown. (D) Confocal microscopic analysis of FITC-DX uptake by fresh RTDCs.
To further confirm the endocytic ability of freshly isolated RTDCs, internalization of FITC-DX was examined by confocal microscopy. At lower magnifications a range of labeling intensities could be observed, with the more strongly endocytic cells appearing to incorporate the label into intracellular vesicles (Fig. 3 D, top), which displayed a peripheral cytoplasmic distribution (Fig. 3 D, bottom). This pattern of vesicle distribution within the cytoplasm was similar to that described for human monocyte-derived DCs (7).
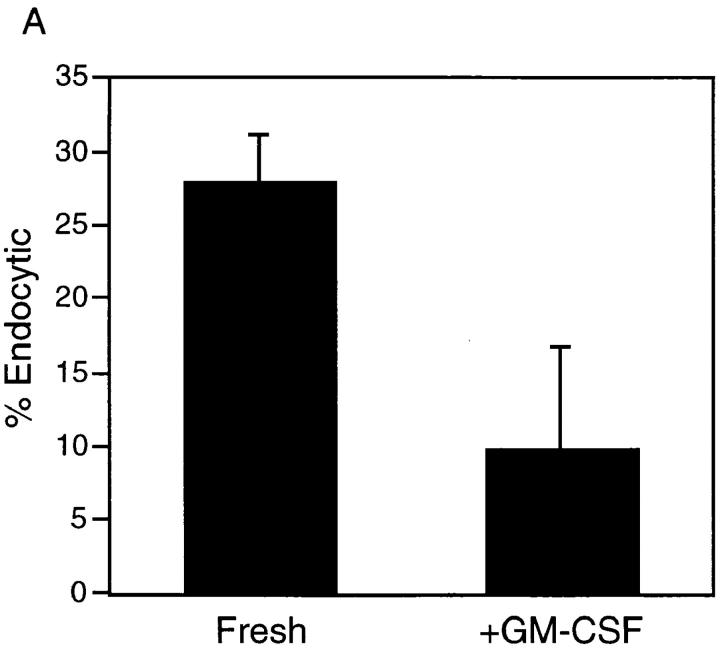
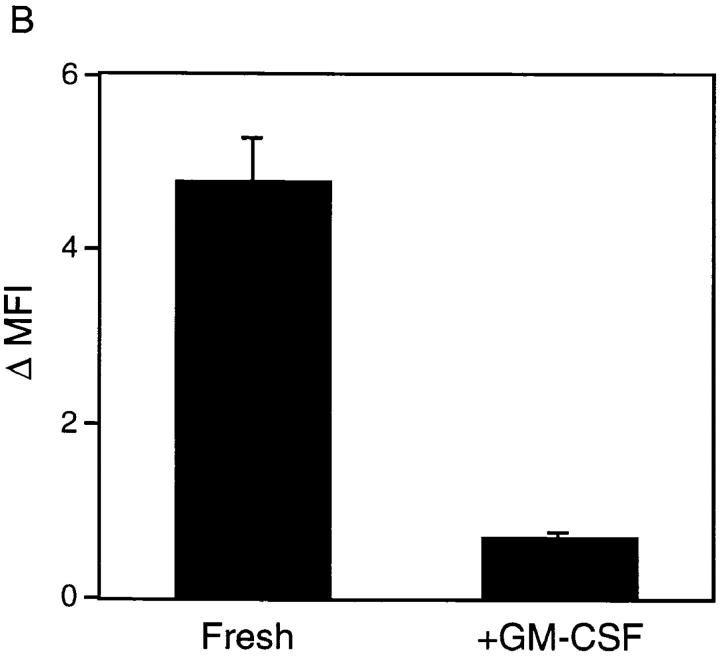
As already noted, studies of DCs isolated from human peripheral blood have described a maturational process whereby endocytic activity is downregulated concomitantly with an upregulation of cell-surface costimulatory molecule expression and T cell–stimulating activity. To determine whether the maturational increases in MHC class II and B7 expression and MLR-stimulating activity described above for RTDCs (Figs. 1 and 2) were also associated with a downregulation of endocytic activity, FITC-DX incorporation by freshly isolated and GM-CSF–matured RTDCs were compared. As shown in Fig. 4, exposure of fresh RTDCs to GM-CSF down-regulated both the percentage of endocytically active cells (Fig. 4 A) and the levels of FITC-DX uptake (Fig. 4 B).
Figure 4.
Inhibition of the endocytic activity of fresh RTDCs by GM-CSF. Fresh or GM-CSF–exposed RTDCs were incubated for 90 min at 37°C with 0.5 mg/ml FITC-DX. Percentage endocytic (A) or Δ MFI (B) values were then calculated from 0°C and 37°C percentage and mean fluorescence values using gating as described in Fig. 3 B. Mean values ± SEM for three experiments are shown.
Taken together, these results demonstrate that freshly isolated RTDCs are actively endocytic and that this endocytic activity is subject to downregulation after in vitro maturation that is associated with an increase in surface MHC class II and B7 expression and in vitro APC activity. Thus, under steady-state conditions, freshly isolated RTDCs appear to be of the immature functional phenotype described for DCs isolated from other sites.
GM-CSF-induced Upregulation of Surface MHC Class II Expression Is Not Associated with Increased mRNA Biosynthesis.
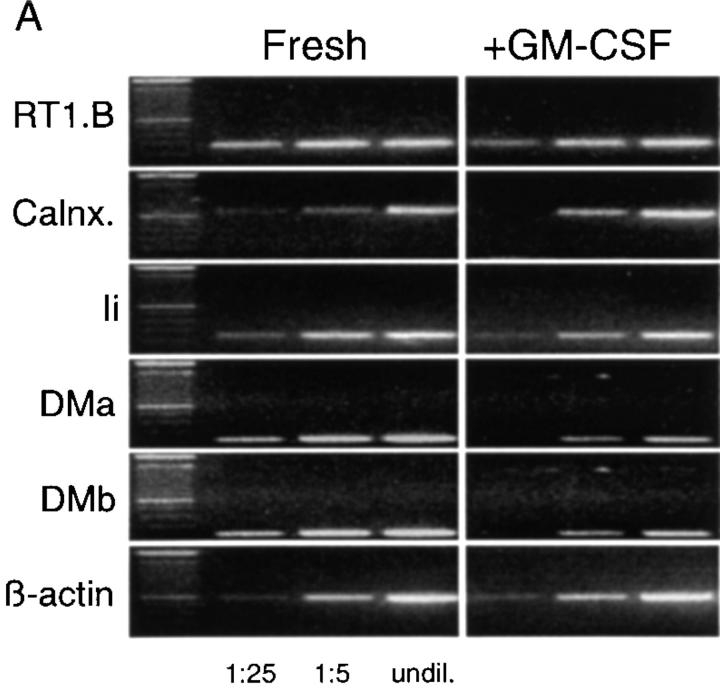
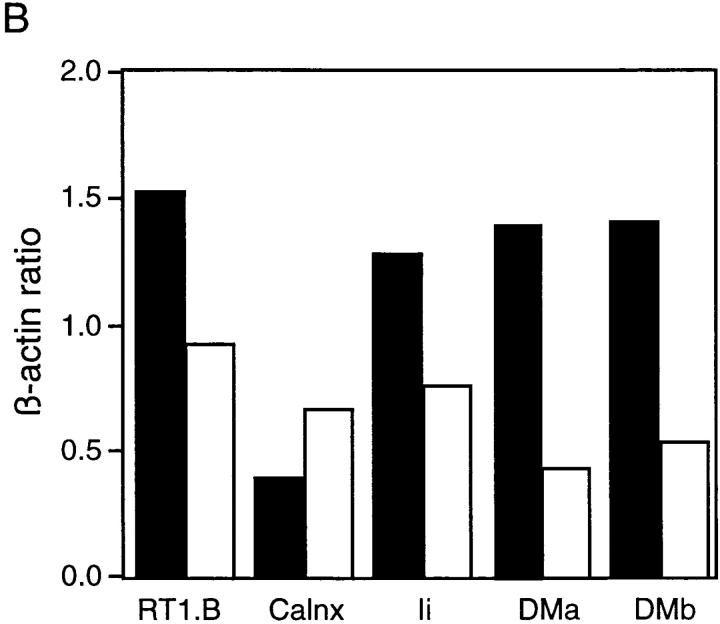
Recently it has been demonstrated that DC maturation leads to stabilization and persistence of MHC class II–peptide complexes on the cell surface, the majority of which are rapidly mobilized from the intracellular pool with only transient increases in de novo protein biosynthesis (39, 40). To determine whether the observed increase in surface expression of MHC class II after exposure of RTDCs to GM-CSF was due to increased biosynthesis of MHC class II protein, changes in expression of mRNA encoding a number of molecules involved in MHC class II biosynthesis and peptide loading were analyzed by reverse transcriptase (RT)-PCR. As shown in Fig. 5, exposure to GM-CSF induced no detectable increase in the levels of expression of mRNA transcripts for RT1.B or the assembly protein calnexin. Furthermore, decreases were observed in the expression of mRNA encoding molecules involved in peptide loading of assembled MHC class II molecules, including invariant chain (Ii) and RT1.DMa and DMb (Fig. 5). These results suggest that the observed increase in surface MHC class II expression on RTDC induced by GM-CSF was not due to increased protein biosynthesis, and are consistent with previous observations indicating that this increased surface protein is mobilized from an intracellular pool. In addition, the decreases in Ii and RT1.DM mRNA expression suggest that mature RTDCs may have a reduced antigen processing/MHC class II peptide loading capacity as described for mature DCs isolated from other sites (38).
Figure 5.
Changes in expression of mRNA encoding components of the MHC class II biosynthesis/processing pathway in fresh or GM-CSF– exposed RTDC. (A) cDNA was prepared from RTDCs either as fresh cells or after overnight culture in GM-CSF and used either undiluted (undil.) or diluted 1:5 or 1:25 in a PCR reaction using primers specific for the indicated MHC class II biosynthesis and antigen processing-associated proteins as described in Materials and Methods. (B) MHC class II component:β-actin mRNA band density ratios (1:5 cDNA dilution) for fresh (solid bars) and GM-CSF–exposed (white bars) RTDCs. One representative out of two experiments is shown. RT1.B, rat MHC class II; Calnx, calnexin; Ii, invariant chain; DMa/DMb, RT1.DMa/b.
Fresh RTDCs Prime for Th2-dependent Antigen-specific IgG Subclass Production.
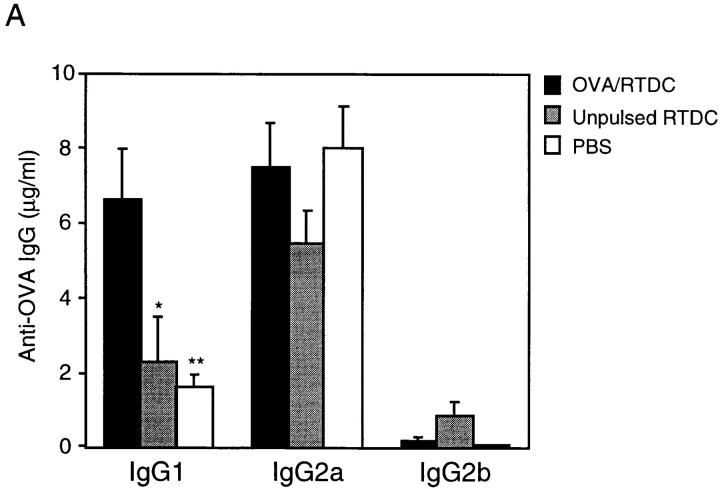
Having established the functional activity of freshly isolated and GM-CSF–matured RTDCs in vitro, we then analyzed the functional properties of these populations of RTDCs in vivo, particularly with respect to their ability to prime for antigen-specific T and B cell responses. Recent reports have demonstrated that DCs pulsed with antigen in vitro act as potent adjuvants when administered in vivo (41), with studies in the mouse showing that splenic DCs are effective at inducing immune responses that involved predominantly Th1-dependent IgG antibodies, accompanied by comparatively low levels of Th2-dependent IgG subclasses (35). We therefore examined the ability of fresh and GM-CSF–matured, OVA-pulsed RTDCs to induce OVA-specific IgG responses in vivo and analyzed the Th-dependent nature of this response in terms of IgG subclass production. Freshly isolated or GM-CSF–exposed RTDCs were incubated at 37°C with 1 mg/ml soluble OVA for 90 min or overnight as described in Materials and Methods, and 105 cells were transferred intravenously to syngeneic recipients. After 5 d, the animals were challenged intravenously with 10 μg/ml OVA in PBS and OVA-specific IgG subclass production was analyzed 15 d later. As shown in Fig. 6 A, animals that received freshly isolated, OVA-pulsed RTDCs produced levels of OVA-specific IgG1 (6.6 ± 1.3 μg/ml) that were significantly greater than those receiving unpulsed RTDCs or PBS (2.2 ± 0.5 and 1.8 ± 0.2 μg/ml; P = 0.01 and 0.005, respectively). In contrast, fresh RTDCs failed to prime for IgG2b production and did not induce significantly raised levels of IgG2a (Fig. 6 A) or IgE (data not shown) when compared with controls.
Figure 6.
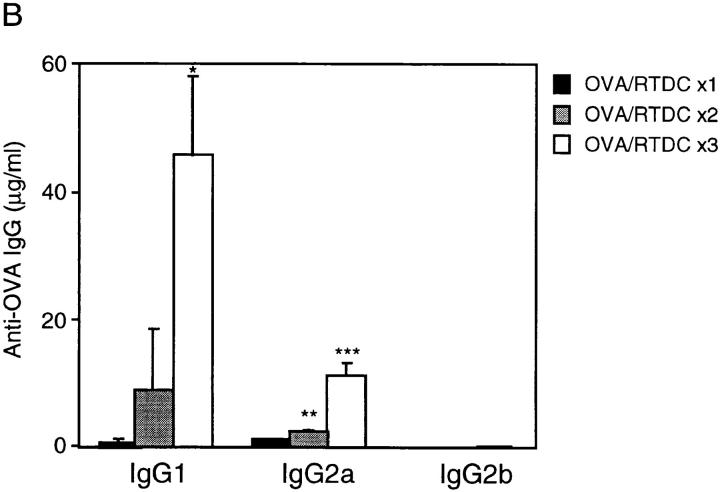
In vivo priming for OVA-specific IgG production by freshly isolated RTDCs. Freshly sorted RTDCs were pulsed with 1 mg/ml OVA for 90 min at 37°C and washed, and 105 cells in 1 ml PBS were transferred intravenously into syngeneic recipients. (A) Animals received a single dose of OVA-pulsed RTDCs on day 0 and were then challenged with 10 μg/ml OVA on day 5 and bled on day 20. Control animals received either 105 unpulsed RTDCs or 1 ml PBS on day 0. *P = 0.01 and **P = 0.005 compared with OVA-pulsed RTDCs. (B) Animals received one dose of RTDCs weekly for 3 wk and were bled 1 wk after final RTDC transfer. *P = 0.005; **P = 0.008; and ***P = 0.01 compared with animals receiving 1× RTDCs. In all cases serum OVA-specific IgG1, 2a, and 2b levels were then assessed by ELISA as described in Materials and Methods. Mean ± SEM of five animals in each group are shown and these experiments have been repeated on several subsequent occasions.
In the rat, IgG1 has been shown to be a Th2-dependent IgG subclass, whereas IgG2b production is indicative of a Th1 response (42, 43). Less direct evidence also suggests that rat IgG2a is Th2 dependent (44, 45). Thus, the above data suggests that freshly isolated RTDCs, through induction of IgG1 but not IgG2b, appeared to prime preferentially for a Th2-dependent antibody response in vivo. Further evidence supporting the Th2-polarizing potential of fresh “resting” RTDCs is shown in Fig. 6 B, where animals repeatedly challenged with OVA-pulsed fresh RTDCs on three occasions at weekly intervals showed increasing levels of IgG1 and IgG2a and not IgG2b, indicating that restriction of the antigen-specific restimulation signal to fresh RTDCs maintains the Th2 polarization of the ensuing antibody response as it matures.
GM-CSF-exposed RTDC Prime for both Th1- and Th2- dependent Antigen-specific IgG Subclass Production.
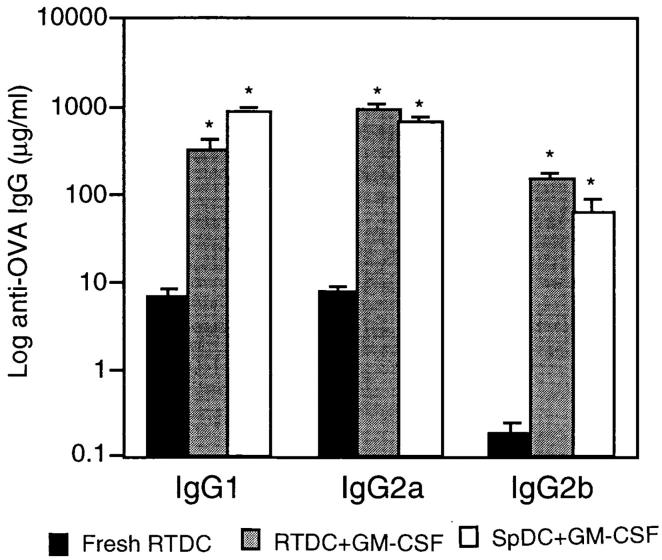
In contrast to the above results obtained with fresh RTDCs, exposure to GM-CSF before transfer dramatically upregulated the in vivo priming activity of RTDCs, with dramatic increases in the production of IgG1 (307 ± 92 μg/ml), IgG2a (894 ± 167 μg/ml), and IgG2b (147 ± 22 μg/ml) when compared with freshly isolated cells indicating increased priming for both Th1- and Th2-dependent responses (Fig. 7). To further support this, analysis of serum IgE demonstrated an increase in IgE titers in recipients of GM-CSF–matured RTDCs, reaching titers equivalent to those obtained after immunization with OVA in the Th2-skewing adjuvant aluminium hydroxide (data not shown). Thus, mature RTDCs now more closely resembled the responses induced by rat splenic-derived DCs, which in this study induced equivalently high levels of all three subclasses, and which, furthermore, are consistent with results reported for mouse splenic-derived DCs (35).
Figure 7.
In vivo priming for OVA-specific IgG production by GM-CSF–exposed RTDCs. Sorted RTDCs or splenic DCs (SpDC) were incubated overnight at 37°C with 1 mg/ml OVA and 10 ng/ml GM-CSF, then transferred intravenously to syngeneic recipients, and serum IgG1, 2a, and 2b levels were determined as described in Fig. 6 A. Identical results for RTDCs were obtained using 90-min OVA-pulsing before overnight culture in GM-CSF (data not shown). Results are expressed as mean ± SEM for 9 (RTDC) or 10 (SpDC) animals. *P < 0.0001 compared with fresh RTDCs.
Induction of OVA-specific T Cell Responses by Fresh and GM-CSF–exposed RTDCs.
To investigate the in vivo T cell priming activity of RTDC preparations, OVA-specific proliferation and IFN-γ production were examined in cultures of spleen cells isolated from adoptive transfer recipients 15 d after the intravenous OVA challenge. As shown in Table 3, freshly isolated OVA-pulsed RTDCs failed to prime for detectable ex vivo OVA-specific spleen cell proliferative responses and induced production of low levels of IL-4, (but not IFN-γ) consistent with the low-level, Th2-skewed IgG production induced by these cells (Fig. 6 A).
Table 3.
Cell Proliferation, IFN-γ, and IL-4 Production in Ex Vivo Spleen Cell Cultures of DC Adoptive Transfer Recipients*
| Type of DC transferred | No. of animals | Proliferation | IFN-γ | IL-4 |
|---|---|---|---|---|
| CPM × 10−3 | U/ml | U/ml | ||
| Fresh RTDCs | 5 | 3.0 ± 0.8 | 0.1 | 1.6 ± 0.39 |
| RTDCs + GM-CSF | 7 | 36.3 ± 2.2 | 10.2 ± 2.5 | 0 |
| Spleen DCs | 9 | 69.4 ± 4.1 | 14.8 ± 3.3 | nt |
| Control** | 8 | 3.8 ± 0.6 | 0.1 | nt |
In contrast, exposure to GM-CSF significantly upregulated the ability of OVA-pulsed RTDCs to prime in vivo for OVA-specific spleen cell responses, inducing a significant degree of ex vivo OVA-specific spleen cell proliferation and high level IFN-γ production (Table 3), consistent with the increased ability of these cells to prime for Th1-dependent IgG2b production (Fig. 7). The inability to detect IL-4 in these cultures, despite the coproduction of Th2-dependent IgG subclasses in these animals, may reflect cytokine consumption in these rapidly proliferating cultures or shutdown (spontaneous or IFN-γ–mediated) of IL-4–secreting cells at this time point (Table 3). OVA-pulsed splenic DCs also induced levels of OVA-specific spleen cell proliferation and IFN-γ production that were of a similar order to that induced by GM-CSF–exposed RTDCs (Table 3), suggesting overall that these two DC types showed similar T cell priming activity in vivo.
RT-PCR Analysis of IL-10 and IL-12 mRNA Production by Fresh and GM-CSF–exposed RTDCs.
To examine whether the functional differences observed between RTDC preparations could be associated with changes in production of immunoregulatory cytokines, semiquantitative RT-PCR analysis for mRNA encoding IL-10 and IL-12 was performed on cDNA isolated from highly purified fresh or GM-CSF–exposed RTDCs. IL-12, a heterodimeric protein consisting of a p35 and p40 chain (46), is one of the major cytokines regulating induction of Th1-mediated responses (23) and has been shown to be produced in large quantities by mature DCs (21, 47). IL-10 has been shown to be produced by several cell types (48), including DCs (49), and is known to be a key regulator of Th2-mediated immune reactions.
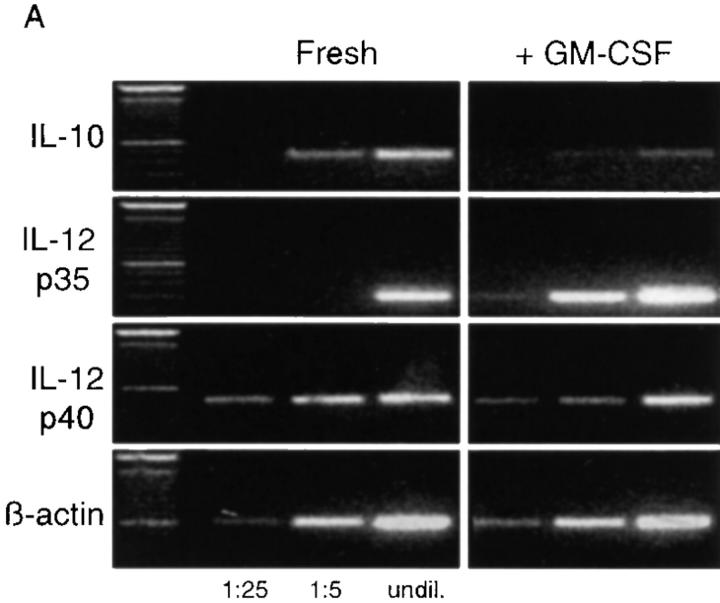
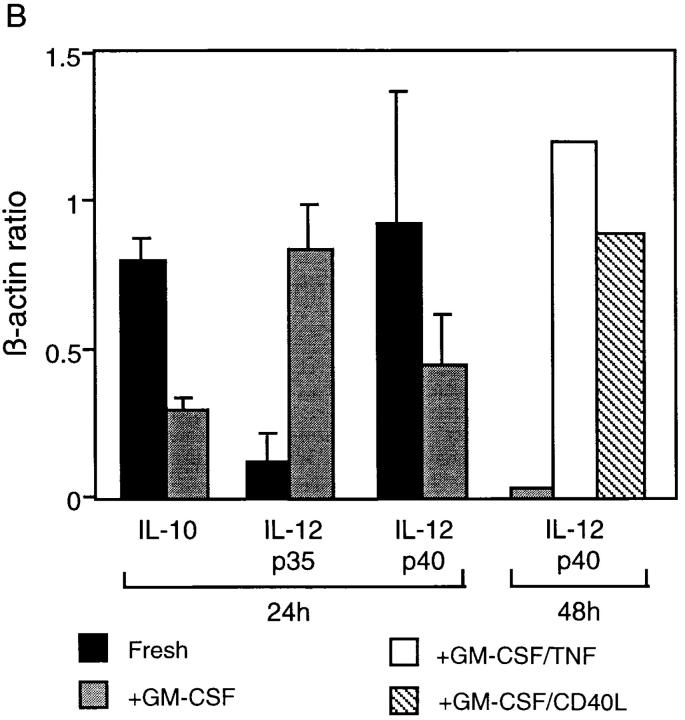
Analysis of cDNA from freshly isolated RTDCs clearly demonstrated the presence of mRNA encoding IL-10, which were downregulated approximately two- to fivefold after exposure to GM-CSF (Fig. 8, A and B). For IL-12, freshly isolated RTDCs expressed low levels of mRNA encoding the p35 chain which were markedly increased after overnight exposure to GM-CSF (Fig. 8 A). Analysis of IL-12p35/β-actin band density ratios indicated that this represented a fourfold increase in the level of mRNA expression (Fig. 8 B). In contrast, mRNA encoding the p40 chain of IL-12 appeared to be highly expressed in freshly isolated cells and generally dropped 30–50% after exposure to GM-CSF (Fig. 8, A and B).
Figure 8.
Expression of IL-10 and IL-12 by fresh and GM-CSF– exposed RTDCs. (A) cDNA prepared from freshly isolated or GM-CSF– exposed RTDCs was used either undiluted (undil.) or diluted 1:5 and 1:25 in PCR reactions for rat IL-10 and IL-12p35 and p40 mRNA as described in Materials and Methods. (B) IL-10/IL-12:β-actin mRNA band density ratios (1:5 cDNA dilution) for fresh RTDCs and RTDCs exposed to GM-CSF alone for 24 or 48 h or GM-CSF plus TNF or CD40L for 48 h only. Mean values ± SEM for three experiments are shown for fresh and GM-CSF–exposed RTDCs, and representative results of a single experiment are shown for GM-CSF plus TNF or CD40L.
These findings suggested that high level expression of IL-12 by RTDCs requires additional maturation stimuli and several laboratories have reported a role for TNF-α and CD40 ligand (CD40L) in this context (50, 51), prompting the experiments shown in Fig. 8 B. These indicate that IL-12 p40 mRNA expression is upregulated in the presence of both TNF-α, and CD40L, but requires 48 h of exposure; IL-12 p35 expression in the same cells maintained the high levels observed in GM-CSF–stimulated cultures at 24 h (data not shown). IL-12 p40 mRNA production was not maintained in 48-h cultures containing GM-CSF alone (Fig. 8 B).
Discussion
Recent studies have established that resident tissue DCs function as “sentinel” cells, being specialized for the acquisition of antigens ranging from soluble protein through to viral particles (15, 52). These and subsequent in vitro studies on the growth of DCs from precursors (53) suggest a complex life history for these cells, involving the progressive transition through functionally distinct phases as they mature. Thus, relatively “immature” DCs from cultures of bone marrow–derived precursors grown in GM-CSF + IL-4 have been suggested to be equivalent to those in peripheral tissues, being specialized for antigen uptake but ineffective as activators of T cells. In contrast, the more mature DCs grown in GM-CSF + TNF-α and CD40L are hypothesized to be equivalent to those residing as interdigitating cells in secondary lymphoid organs, displaying low capacity for antigen uptake but potent T cell activating function (38). However, it is not known precisely how applicable this model is to DC populations in vivo, particularly to those at mucosal surfaces such as RTDCs, which display steady-state turnover rates up to 10 times faster than more studied DC populations such as epidermal Langerhans cells (24).
This study therefore sought to characterize in detail the functional phenotype of RTDCs in resting tissue and to determine the degree to which their function(s) can be modulated via stimuli previously identified as promoters of DC maturation. The overall aim of this study was to elucidate the role of RTDCs in Th1/Th2 regulation of immune responses to antigens impacting on the airway mucosa, and accordingly a key element of these experiments involved analysis of the in vivo T cell priming properties of RTDCs after adoptive transfer.
The salient findings from these experiments are as follows. First, it is clear that a major subset of resident RTDCs express surface phenotypic features comparable to “immature” DCs grown in vitro from blood- and bone marrow– derived precursors, particularly expression of low-moderate levels of MHC class I and II and also CD80/86, coupled with high endocytic activity and poor capacity to stimulate MLR responses. However, significant heterogeneity exists with respect to intensity of expression of several markers, in particular MHC class I and II and CD4. Ultra high level MHC class II expression was limited to a minor subset of freshly isolated RTDCs and these cells were nonendocytic (Fig. 3), suggesting possible maturation in situ (see below). We plan future follow-up studies on subsets defined by intensity of expression of these markers.
It is additionally clear that culture with GM-CSF elicits a series of changes associated with functional upregulation, notably increased expression of MHC class I and II and CD80/86 together with a loss of endocytic activity, a decrease in expression of a series of genes encoding molecules involved in antigen processing, and a concomitant increase in MLR-stimulatory activity. CD4 expression was also lost (Table 2) and the significance of this observation will be explored in later studies.
Thus, respiratory mucosal surfaces are endowed with a resident population of DCs that are highly specialized for uptake and processing of antigen, and capable of rapid maturation into potent APCs upon receipt of appropriate cytokine signals. The heterogeneity observed, particularly with respect to MHC class II expression and levels of endocytic activity, suggests that the population overall represents a developmental continuum that is skewed heavily (at least in the steady state) towards the “immature” functional phenotype. It will be of interest to determine whether this activity spectrum changes with acute and chronic stimulation, given recent findings indicating the uniquely dynamic features of the RTDC populations under conditions of local stress (5, 25, 54).
Although well characterized in vitro, the functional activity of DC in vivo, particularly in terms of their ability to activate Th1- or Th2-mediated T cell responses, still remains unclear to a large extent. The primary role of these cells is thought to involve protection against infectious agents (19, 55) through strong promotion of Th1-dependent immunity, primarily via production of high levels of IL-12 (21, 50, 56). However, as noted above, studies focussing on mucosal surfaces such as the lung and gut clearly indicate that the baseline response to inert antigens at these sites displays a Th2 bias. Accordingly, a key aim of this study was to determine the relationship between the ex vivo phenotype of RTDCs and their in vivo functions, in the context of Th1/2 switch regulation. The experiments reported in Fig. 6 A demonstrate that freshly isolated RTDCs are able to prime T helper cells in adoptive recipients, but the priming is relatively weak and restricted to Th2-dependent IgG1. The experiments of Fig. 6 B provide further evidence of Th2 bias on the part of these RTDCs, via the demonstration that three successive challenges with antigen-pulsed DCs progressively expands the Th2-dependent IgG subclass component of these responses (IgG1 and IgG2a), with minimal effects on Th1-dependent IgG2b production.
In contrast, GM-CSF–matured RTDCs display a 1–2 log increase in overall priming capacity, which includes a major component of Th1-dependent IgG2b, and this pattern was identical to that seen with splenic DCs (Fig. 7), and is consistent with earlier reports on mouse splenic DCs (14, 35). Thus, it appears that resident RTDCs under steady-state conditions are programmed for induction of Th2-biased responses, and require endogenous activation/ maturation signals in order to develop capacity to stimulate Th1 immunity. A possible explanation for these findings was provided by RT-PCR analysis of cytokine production, which demonstrated expression of mRNA encoding IL-10 in fresh RTDCs (Fig. 8). IL-10 is known to have potent immune-modulating effects (57, 58) and acts to preferentially inhibit Th1 priming. Thus, IL-10 inhibits IL-12 production by DCs (47) and pretreatment of DCs with IL-10 has been shown to suppress their ability to stimulate Th1 responses both in vitro and in vivo (59–62). In addition, treatment of DCs with PGE2, a Th2-promoting inflammatory mediator, downregulates IL-12 and upregulates IL-10 production and the in vitro Th2-stimulating capacity of these cells (49). Consistent with these observations, fresh RTDCs appeared to produce mRNA for both subunits of IL-12, but the available data suggests that assembly of the functional heterodimer in these cells may be severely limited by the restricted levels of p35 subunit available (Fig. 8).
Recent evidence also suggests that IL-10 acts posttranslationally to inhibit surface expression of MHC class II (63), and thus IL-10 production by fresh RTDCs may contribute to the low steady-state expression of this marker on the majority of these cells. This may serve to limit the magnitude of the antigen signal delivered to T cells to the (low) dose range that preferentially promotes Th2-dominated responses (64). Moreover, IL-10 has been demonstrated to suppress CD80 expression (65), and its production by fresh RTDCs may thus contribute towards selective expression on these cells of CD86 (Fig. 2). It has been suggested that costimulation through CD86 preferentially activates Th2 immunity (66), although this issue remains controversial (67).
IL-10 at relatively high concentrations has also been demonstrated to exert a generalized inhibitory effect on T cell proliferation (48), and it is accordingly possible that IL-10 production by fresh RTDCs may act to limit the overall size of the primary immune responses that they induce.
Thus, autocrine production of IL-10 by RTDCs may contribute towards restriction of the T cell priming activity of these cells to the promotion of (initially) low level Th2-biased responses, via a number of mechanisms. Consistent with this hypothesis, exposure of RTDC to GM-CSF markedly upregulates both Th2 and Th1 priming capacity, and this is accompanied by concomitant downregulation of IL-10 mRNA production and increased production of IL-12 p35 mRNA (Fig. 8). Although the production of IL-12 p40 mRNA remained low, the observation that GM-CSF– exposed RTDCs behaved in vivo in a functionally similar fashion to rat (Fig. 7) and mouse (35) splenic DCs, being strong inducers of both Th1- and Th2-mediated responses, indicated that functionally active IL-12 is likely to have been produced in vivo. This suggests that the levels of IL-12p35 and IL-12p40 produced by GM-CSF–treated RTDCs are sufficient to yield biologically significant amounts of IL-12 heterodimer, and/or that stimulatory factors in addition to GM-CSF (for example CD40L; reference 50) become available to the RTDCs after adoptive transfer.
Collectively, these findings provide a plausible explanation for the constitutive Th2 bias operative in mucosal immune responses to inert protein antigens, which is amenable to further testing. In particular, it will be important to formally demonstrate the production of bioactive IL-10 and IL-12 protein by RTDCs, and this issue will be addressed in future experiments. The underlying mechanism(s) described above may also play a significant role in the progressive deviation of immune responses to airborne protein antigens from initial Th2 to eventual Th1 polarity, a phenomenon that has been described in detail in both animal model experiments and in humans undergoing chronic exposure to inhaled antigen (68). We speculate that other classes of antigen that induce local inflammation in the airway mucosa, particularly microbial antigens, would elicit responses with a much large Th1 component as a result of the effects of inflammation- associated cytokines on RTDCs.
Additionally, the above results suggest new avenues for research into the aetiology and pathogenesis of respiratory allergy. Two distinct phases are now recognized in these diseases in humans. The first involves a failure of immune deviation mechanisms that normally bias immune responses against inhaled protein antigens towards Th1, resulting in the development of potentially pathogenic Th2-polarized memory and the active production of specific IgE antibody (69). The second phase, which occurs in only a subset of subjects who develop Th2 memory against these inhaled antigens, involves chronic airways inflammation characterized by the presence of large numbers of activated T cells (70), which infers the presence in the airway mucosa of functionally mature APCs. It is of interest to note in this context that local airway intraepithelial production of high levels of GM-CSF is a hallmark of atopic asthma (71). It is also relevant to note recent studies that suggest that peripheral tissue DC populations comprise two distinct myeloid lineages, one of which is characterized by high endocytic activity/IL-10 production/capacity to induce naive B cell switching, and the second being specialized for T cell activation (72, 73). The balance between these two subpopulations in the airway mucosa in the steady state and during inflammation may be an important determinant of local immune responder phenotype. The principal focus of ongoing studies in this model will accordingly be upon RTDC heterogeneity.
Acknowledgments
We thank Dr. Andrew McWilliam for help with design of PCR primers.
This work was supported by grants from the National Health and Medical Research Council of Australia and Glaxo-Wellcome Australia.
Abbreviations used in this paper
DC
dendritic cell
GAM-PE
PE-conjugated goat anti–mouse IgG
GKN
solution consisting of 11 mM d-glucose, 5.5 mM KCl, 137 mM NaCl, 25 mM Na2HPO4, and 5.5 mM Na2HPO4 × 2H2O
mφ
macrophages
RTDC
respiratory tract DC
RT-PCR
reverse transcriptase PCR
References
- 1.Holt PG, Schon-Hegrad MA, Oliver J. MHC class II antigen-bearing dendritic cells in pulmonary tissues of the rat. Regulation of antigen presentation activity by endogenous macrophage populations. J Exp Med. 1988;167:262–274. doi: 10.1084/jem.167.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holt PG, Schon-Hegrad MA, Phillips MJ, McMenamin PG. Ia-positive dendritic cells form a tightly meshed network within the human airway epithelium. Clin Exp Allergy. 1989;19:597–601. doi: 10.1111/j.1365-2222.1989.tb02752.x. [DOI] [PubMed] [Google Scholar]
- 3.Nicod LP, Lipscomb MF, Weissler JC, Lyons CR, Albertson J, Toews GB. Mononuclear cells in human lung parenchyma. Characterization of a potent accessory cell not obtained by bronchoalveolar lavage. Am Rev Respir Dis. 1987;136:818–823. doi: 10.1164/ajrccm/136.4.818. [DOI] [PubMed] [Google Scholar]
- 4.Pollard AM, Lipscomb MF. Characterization of murine lung dendritic cells: similarities to Langerhans cells and thymic dendritic cells. J Exp Med. 1990;172:159–167. doi: 10.1084/jem.172.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schon-Hegrad MA, Oliver J, McMenamin PG, Holt PG. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med. 1991;173:1345–1356. doi: 10.1084/jem.173.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine TP, Chain BM. Endocytosis by antigen presenting cells: dendritic cells are as endocytically active as other antigen presenting cells. Proc Natl Acad Sci USA. 1992;89:8342–8346. doi: 10.1073/pnas.89.17.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen CP, Ritchie SC, Pearson TC, Linsley PS, Lowry RP. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992;176:1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young JW, Koulova L, Soergel SA, Clark EA, Steinman RM, Dupont B. The B7/BB1 antigen provides one of several costimulatory signals for the activation of CD4+T lymphocytes by human blood dendritic cells in vitro. J Clin Invest. 1992;90:229–237. doi: 10.1172/JCI115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nussenzweig MC, Steinman RM. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med. 1980;151:1196–1212. doi: 10.1084/jem.151.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason DW, Pugh CW, Webb M. The rat mixed lymphocyte reaction: roles of a dendritic cell in intestinal lymph and T-cell subsets defined by monoclonal antibodies. Immunology. 1981;44:75–87. [PMC free article] [PubMed] [Google Scholar]
- 12.Macatonia SE, Taylor PM, Knight SC, Askonas BA. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989;169:1255–1264. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sornasse T, Flamand V, De Becker G, Bazin H, Tielemans F, Thielemans K, Urbain J, Leo O, Moser M. Antigen-pulsed dendritic cells can efficiently induce an antibody response in vivo. J Exp Med. 1992;175:15–21. doi: 10.1084/jem.175.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romani N, Koide S, Crowley M, Witmer-Pack M, Livingstone AM, Fathman CG, Inaba K, Steinman RM. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J Exp Med. 1989;169:1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Streilein JW, Grammer SF. In vitro evidence that Langerhans cells can adopt two functionally distinct forms capable of antigen presentation to T lymphocytes. J Immunol. 1989;143:3925–3933. [PubMed] [Google Scholar]
- 17.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann VS, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 19.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 20.Macatonia SE, Hsieh CS, Murphy KM, O'Garra A. Dendritic cells and macrophages are required for Th1 development of CD4+T cells from alpha beta TCR transgenic mice: IL-12 substitution for macrophages to stimulate IFN-gamma production is IFN-gamma-dependent. Int Immunol. 1993;5:1119–1128. doi: 10.1093/intimm/5.9.1119. [DOI] [PubMed] [Google Scholar]
- 21.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 22.Hilkens CM, Kalinski P, de Boer M, Kapsenberg ML. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997;90:1920–1926. [PubMed] [Google Scholar]
- 23.Trinchieri G, Scott P. The role of interleukin 12 in the immune response, disease and therapy. Immunol Today. 1994;15:460–463. doi: 10.1016/0167-5699(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 24.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol. 1994;153:256–261. [PubMed] [Google Scholar]
- 25.McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179:1331–1336. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacPherson GG, Jenkins CD, Stein MJ, Edwards C. Endotoxin-mediated dendritic cell release from the intestine. Characterization of released dendritic cells and TNF dependence. J Immunol. 1995;154:1317–1322. [PubMed] [Google Scholar]
- 27.Holt P, Batty J, Turner K. Inhibition of specific IgE responses in mice by specific exposure to inhaled antigen. Immunology. 1981;42:409–417. [PMC free article] [PubMed] [Google Scholar]
- 28.Sedgwick JD, Holt PG. Induction of IgE- secreting cells and IgE isotype-specific suppressor T cells in the respiratory lymph nodes of rats in response to antigen inhalation. Cell Immunol. 1985;94:182–194. doi: 10.1016/0008-8749(85)90095-4. [DOI] [PubMed] [Google Scholar]
- 29.McMenamin C, Holt PG. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I–restricted CD8+ T cell–mediated but MHC class II–restricted CD4+T cell– dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993;178:889–899. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoyne GF, Asknonas BA, Hetzel C, Thomas WR, Lamb JR. Regulation of house dust mite responses by inhaled peptides: transient activation precedes the development of tolerance in vivo. . Int Immunol. 1996;8:335–342. doi: 10.1093/intimm/8.3.335. [DOI] [PubMed] [Google Scholar]
- 31.Wells HG, Osborne TB. The biological reactions of the vegetable proteins. J Infect Dis. 1911;8:77–82. [Google Scholar]
- 32.Chase MW. Inhibition of experimental drug allergy by prior feeding of the sensitizing agent. Proc Soc Exp Biol (NY) 1946;61:257–259. doi: 10.3181/00379727-61-15294p. [DOI] [PubMed] [Google Scholar]
- 33.Weiner HL, Friedman A, Miller A, Khoury SJ, al-Sabbagh A, Santos L, Sayegh M, Nussenblatt RB, Trentham DE, Hafler DA. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 34.Daynes RA, Araneo BA, Dowell TA, Huang K, Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990;171:979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeBecker G, Sornasse T, Nabavi N, Bazin H, Tielemans F, Urbain J, Leo O, Moser M. Immunoglobulin isotype regulation by antigen-presenting cells in vivo. Eur J Immunol. 1994;24:1523–1528. doi: 10.1002/eji.1830240710. [DOI] [PubMed] [Google Scholar]
- 36.Sedgwick JD, Holt PG. Induction of IgE-isotype specific tolerance by passive antigenic stimulation of the respiratory mucosa. Immunology. 1983;50:625–630. [PMC free article] [PubMed] [Google Scholar]
- 37.Stumbles P, Mason D. Activation of CD4+T cells in the presence of a nondepleting monoclonal antibody to CD4 induces a Th2-type response in vitro. J Exp Med. 1995;182:5–13. doi: 10.1084/jem.182.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 39.Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman RM, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 40.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 41.Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saoudi A, Kuhn J, Huygen K, de Kozak Y, Velu T, Goldman M, Druet P, Bellon B. TH2 activated cells prevent experimental autoimmune uveoretinitis, a TH1- dependent autoimmune disease. Eur J Immunol. 1993;23:3096–3103. doi: 10.1002/eji.1830231208. [DOI] [PubMed] [Google Scholar]
- 43.Gracie JA, Bradley JA. Interleukin-12 induces interferon-gamma-dependent switching of IgG alloantibody subclass. Eur J Immunol. 1996;26:1217–1221. doi: 10.1002/eji.1830260605. [DOI] [PubMed] [Google Scholar]
- 44.Der Balian, G., J. Slack, B.L. Clevinger, H. Basin, and J.M. Davie. Subclass restriction of murine antibodies. III. Antigens that stimulate IgG3 in mice stimulate IgG2cin rats. J Exp Med. 1980;152:209–218. doi: 10.1084/jem.152.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuturi MC, Josien R, Cantarovich D, Bugeon L, Anegon I, Menoret S, Smit H, Douillard P, Soulillou JP. Decreased anti-donor major histocompatibility complex class I and increased class II alloantibody response in allograft tolerance in adult rats. Eur J Immunol. 1994;24:1627–1631. doi: 10.1002/eji.1830240726. [DOI] [PubMed] [Google Scholar]
- 46.Yoshimoto T, Kojima K, Funakoshi T, Endo Y, Fujita T, Nariuchi H. Molecular cloning and characterization of murine IL-12 genes. J Immunol. 1996;156:1082–1088. [PubMed] [Google Scholar]
- 47.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore KW, O'Garra A, de Waal R, Malefyt, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 49.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 50.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Austyn JM. New insights into the mobilization and phagocytic activity of dendritic cells. J Exp Med. 1996;183:1287–1292. doi: 10.1084/jem.183.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nonacs R, Humborg C, Tam JP, Steinman RM. Mechanisms of mouse spleen dendritic cell function in the generation of influenza-specific, cytolytic T lymphocytes. J Exp Med. 1992;176:519–529. doi: 10.1084/jem.176.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McWilliam AS, Marsh AM, Holt PG. Inflammatory infiltration of the upper airway epithelium during Sendai virus infection: involvement of epithelial dendritic cells. J Virol. 1997;71:226–236. doi: 10.1128/jvi.71.1.226-236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 56.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 57.de Waal Malefyt, R., J. Haanen, H. Spits, M.G. Roncarolo, A. te Velde, C. Figdor, K. Johnson, R. Kastelein, H. Yssel, and J.E. de Vries. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Grazia M, Roncarlo A CD4+T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 59.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–1235. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 60.Enk AH, Angeloni VL, Udey MC, Katz SI. Inhibition of Langerhans cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J Immunol. 1993;151:2390–2398. [PubMed] [Google Scholar]
- 61.Macatonia SE, Doherty TM, Knight SC, O'Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. 1993;150:3755–3765. [PubMed] [Google Scholar]
- 62.Qin Z, Noffz G, Mohaupt M, Blankenstein T. Interleukin-10 prevents dendritic cell accumulation and vaccination with granulocyte-macrophage colony-stimulating factor gene-modified tumor cells. J Immunol. 1997;159:770–776. [PubMed] [Google Scholar]
- 63.Koppelman B, Neefjes JJ, deVries JE, de Waal R, Malefyt Interleukin-10 down-regulates MHC class II αβ peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7:861–871. doi: 10.1016/s1074-7613(00)80404-5. [DOI] [PubMed] [Google Scholar]
- 64.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ozawa H, Aiba S, Nakagawa H, Tagami H. Interferon-gamma and interleukin-10 inhibit antigen presentation by Langerhans cells for T helper type 1 cells by suppressing their CD80 (B7-1) expression. Eur J Immunol. 1996;26:648–652. doi: 10.1002/eji.1830260321. [DOI] [PubMed] [Google Scholar]
- 66.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: applications to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 67.Schweitzer AN, Borriello F, Wong RC, Abbas AK, Sharpe AH. Role of costimulators in T cell differentiation: studies using antigen-presenting cells lacking expression of CD80 or CD86. J Immunol. 1997;158:2713–2722. [PubMed] [Google Scholar]
- 68.Holt PG. Immunoprophylaxis of atopy: light at the end of the tunnel? . Immunol Today. 1994;15:484–489. doi: 10.1016/0167-5699(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 69.Holt PG, Macaubas C. Development of long-term tolerance versus sensitisation to environmental allergens during the perinatal period. Curr Opin Immunol. 1997;9:782–787. doi: 10.1016/s0952-7915(97)80178-1. [DOI] [PubMed] [Google Scholar]
- 70.Corrigan CJ, Kay AB. T-cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992;13:501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- 71.Poston RN, Chanez P, Lacoste JY, Litchfield T, Lee TH, Bousquet J. Immunohistochemical characterization of the cellular infiltrate in asthmatic bronchi. Am Rev Respir Dis. 1992;145:918–921. doi: 10.1164/ajrccm/145.4_Pt_1.918. [DOI] [PubMed] [Google Scholar]
- 72.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor γ. II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 73.de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Aït-Yahia S, Banchereau J, Liu Y-J, Lebecque S, Caux C. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160:1666–1676. [PubMed] [Google Scholar]