A MADS Box Protein Consensus Binding Site Is Necessary and Sufficient for Activation of the Opaque-Phase-Specific Gene OP4 of Candida albicans (original) (raw)
Abstract
The majority of strains of Candida albicans can switch frequently and reversibly between two or more general phenotypes, a process now considered a putative virulence factor in this species. Candida albicans WO-1 switches frequently and reversibly between a white and an opaque phase, and this phenotypic transition is accompanied by the differential expression of white-phase-specific and opaque-phase-specific genes. In the opaque phase, cells differentially express the gene OP4, which encodes a putative protein 402 amino acids in length that contains a highly hydrophobic amino-terminal sequence and a carboxy-terminal sequence with a pI of 10.73. A series of deletion constructs fused to the Renilla reniformis luciferase was used to functionally characterize the OP4 promoter in order to investigate how this gene is differentially expressed in the white-opaque transition. An extremely strong 17-bp transcription activation sequence was identified between −422 and −404 bp. This sequence contained a MADS box consensus binding site, most closely related to the Mcm1 binding site of Saccharomyces cerevisiae. A number of point mutations generated in the MADS box consensus binding site as well as a complete deletion of the consensus site further demonstrated that it was essential for the activation of OP4 transcription in the opaque phase. Gel mobility shift assays with the 17-bp activation sequence identified three specific complexes which formed with both white- and opaque-phase cell extracts. Competition with a putative MADS box consensus binding site from the promoter of the coordinately regulated opaque-phase-specific gene PEP1 (SAP1) and the human MADS box consensus binding site for serum response factor demonstrated that one of the three complexes formed was specific to the OP4 sequence.
Candida albicans and a number of related species undergo high-frequency phenotypic switching between a number of general phenotypes distinguishable, in most cases, by differences in colony morphology and, in some cases, by differences in cellular morphology (36, 42, 43, 45, 48, 49). Switching represents a developmental program distinct from the bud-hypha transition, although some of the same characteristics, including the expression of particular phase-specific genes, are regulated by both programs (4, 45, 55). Since switching has been demonstrated to affect a number of different putative virulence factors (4, 21, 22, 30, 44, 45, 57), including expression of the drug-resistant gene CDR3 (6), it has been suggested that it represents, in its own right, a virulence factor that provides the cell with the ability to respond rapidly to changes in its environment (46). Different strains of C. albicans may express different repertoires of colony morphologies, but the general characteristics of the switching process in all strains tested are surprisingly similar (45), and some of the same genes are regulated by switching in different strains (32, 45, 49). To understand how switching is regulated, a reverse-genetics approach has been undertaken in which the regulatory circuitry which controls the expression of phase-specific genes is being tracked upstream with the expectation that it will lead to the switch event itself (48, 49). In this pursuit, the white-opaque transition in C. albicans WO-1 (43) has been used as a model system since it involves a simple and reversible transition between a white- and an opaque-colony-forming phenotype that is accompanied by dramatic alterations in cellular morphology and physiology (3, 5, 38, 44, 45). It has been demonstrated that the white-opaque transition is accompanied by the differential expression of phase-specific genes (30, 31, 44, 48, 49, 51). Transcriptional regulation of the white-phase-specific gene WH11 (51) has been demonstrated to be effected through two strong and one weak transcription activation sequence in the promoter, and gel retardation experiments suggest that white-phase-specific transcription activation factors interact with the two strong activation sequences (52, 55).
Here, we have functionally characterized for the first time the promoter of an opaque-phase-specific gene, OP4 (31). OP4 is differentially expressed in the opaque phase of the white-opaque transition in strain WO-1; it is silent in both the budding and the hyphal phenotypes of the white phase (31). OP4 is also differentially expressed in the variant phenotypes, but not in the basic smooth white phenotype, of the switching repertoire of the common laboratory strain 3153A (32). OP4 encodes a putative protein (31) containing 402 amino acids that has no significant homology with any protein described to date in available computer databases. OP4 contains a hydrophobic amino terminus of 26 amino acids, a pI of 10.73 for the last 100 amino acids, two serine repeats adjacent to alanine repeats, and possible α-helical conformation within the alanine-rich sequences. The OP4 promoter functions ectopically in a phase-specific manner (54) and has been used to misexpress the white-phase-specific gene WH11 in the opaque phase (26). To understand how OP4 is regulated in the white-opaque transition, the promoter has been functionally characterized by fusing deletion and point mutation constructs of the promoter to the luciferase gene RLUC of the sea pansy Renilla reniformis, an effective reporter in C. albicans (54), and analyzing transformants for Rluc activity in the white and opaque phases. Phase-specific factors which may interact with regulatory sequences have been investigated by gel retardation experiments. It is demonstrated that OP4 expression is regulated primarily by a strong transcription activation sequence which consists almost exclusively of a MADS box consensus binding site with strong homology to the Mcm1 binding site of Saccharomyces cerevisiae (1, 2).
MATERIALS AND METHODS
Maintenance of stock cultures.
Red 3/6, an ade2 auxotrophic derivative (56) of C. albicans WO-1 (43), was stored at −80°C. For experimental purposes, cells from stock cultures were plated on agar containing supplemented Lee’s medium (7) with 0.6 μM adenine sulfate (52). Transformants which acquired adenine prototrophy were maintained in the absence of adenine sulfate. White- and opaque-phase cells were obtained by growing cells from a white- or an opaque-phase colony, respectively, in supplemented Lee’s liquid medium without adenine sulfate at 25°C. The proportions of white-phase and opaque-phase cells were determined microscopically for each cell population (3, 5, 43), and only populations which contained >97% of the expected cell type were used for biochemical analysis.
Construction of promoter derivatives.
Deletion and mutation derivatives of the OP4 promoter were inserted at the multiple cloning site immediately upstream of the open reading frame of the R. reniformis luciferase gene (RLUC) in the transforming plasmid pCRW3, which contains the C. albicans ADE2 gene (54). Construction of the plasmid pCROP31, which contained the 854-bp region upstream of OP4, has been described in detail in a previous study (54). Deletion and mutation derivatives were derived by using the oligonucleotide primers described in Fig. 1 to generate PCR products with a λ clone containing a 10-kb genomic fragment of OP4 (31) as template (Table 1). All deletion derivatives were sequenced. The PCR products were inserted into plasmid pCRW3 either as a _Pst_I fragment (CROP82, CROP83, CROP84, and CROP85) or as a _Pst_I/_Sma_I fragment (CROP12, CROP9, CROP86, CROP7, M1, M3, M5, M6, M7, M8, M9, M10, and M11). CROP23 was generated by cleaving pCROP31 with _Sac_I and religating the plasmid to create a 115-bp deletion. pOΔMADS was created by cleaving CROP85 with _Sma_I and _Sal_I in the polylinker and then directionally inserting PCR fragment CROP32. pOWhyb was created by directionally inserting PCR fragment WHM4A at the _Afl_II site of plasmid pCRW5ΔAf (55), which contains the transcription start site of the white-phase-specific gene WH11 upstream of RLUC. To generate point mutations, oligonucleotides carrying one or more specific point mutations of the OP4 promoter were used in PCRs with λOP4 as template. All point mutations were confirmed by sequencing.
FIG. 1.
Oligonucleotides used for generating deletion constructs and for gel mobility shift assays.
TABLE 1.
Oligonucleotides used to make the various PCR products used in this study
| Product | Oligonucleotide | |
|---|---|---|
| 5′ | 3′ | |
| CROP31 | 2.1 | 2.2 |
| CROP12 | U12 | 2.2 |
| CROP9 | U9 | 2.2 |
| CROP82 | U82 | 2.2 |
| CROP83 | U83 | 2.2 |
| CROP84 | U84 | 2.2 |
| CROP85 | U85 | 2.2 |
| CROP86 | U86 | 2.2 |
| CROP7 | U7 | 2.2 |
| M1 | M1 | 2.2 |
| M3 | M3 | 2.2 |
| M5 | M5 | 2.2 |
| M6 | M6 | 2.2 |
| M7 | M7 | 2.2 |
| M8 | M8 | 2.2 |
| M9 | M9 | 2.2 |
| M10 | M10 | 2.2 |
| M11 | M11 | 2.2 |
| CROP32 | OP5A | OP32B |
| WHM4A | OP5B | M4C |
For integrative transformation of C. albicans, 20 μg of each plasmid was linearized within the ADE2 gene with _Nsi_I. The details of integration at the ade2 locus of strain Red 3/6 have been described in detail in earlier publications (52–54). Transformations were performed by the lithium acetate method as described by Schiestl and Geitz (40). Each transformant was analyzed by Southern blot analysis, and only those that contained a single insertion at the ade2 locus were used in subsequent analyses.
Southern blot analysis.
Total cellular DNA was extracted from C. albicans isolates according to the method of Scherer and Stevens (39). Approximately 5 μg of DNA from transformant clones was digested with either _Bam_HI or _Eco_RI, electrophoresed in a 0.8% agarose gel, transferred to a Nitropure membrane (MSI, Westborough, Mass.) and probed with 32P-labeled ADE2. Blots were washed (12) and exposed to X-OMAT X-ray film (Kodak, Inc., Rochester, N.Y.) at −70°C.
Northern blot analysis.
Methods for the isolation of total cell RNA and Northern blot hybridization have been described in earlier publications (30, 51). Blots were first probed with 32P-labeled OP4 and autoradiographed, then stripped, hybridized with 32P-labeled C. albicans ADE2, a constitutively expressed gene, and autoradiographed again.
In vitro luciferase assays.
C. albicans cells were grown to late log phase in supplemented Lee’s liquid medium (7). Cells (2 × 108) were washed once with sterile distilled water and once with luciferase buffer (0.5 M NaCl, 0.1 M K2HPO4 [pH 6.7], 1 mM EDTA, 0.6 mM sodium azide, 1 mM phenylmethylsulfonyl fluoride, 0.02% bovine serum albumin) (29). The cell pellet was mixed with an equal volume of glass beads (0.45-μm diameter) and 200 μl of luciferase buffer and lysed with a bead beater (Biospec Products, Bartlesville, Okla.) through five cycles of 30-s duration, with intervening 1-min periods of no agitation in an ice bath. One hundred microliters of luciferase buffer containing 0.5 μM coelenterazine (Molecular Probes Inc., Eugene, Oreg.) was mixed with either 2 μl of undiluted cell extract, 2 μl of cell extract diluted 1:10 with luciferase buffer, or 2 μl of cell extract diluted 1:100 with luciferase buffer, in a 4-ml analysis tube (Analytical Luminescence Laboratory, Ann Arbor, Mich.). The coelenterazine was stored in acid-methanol at −20°C. Immediately after cell extract and reaction buffer were mixed, light emission was measured at 480 nm in the integration mode for 30 s in a Monolight 2010 luminometer (Analytical Luminescence Laboratory). Relative light units (RLUs) are presented as light emitted per 30 s per microgram of protein. Protein concentrations were measured by the Bradford assay (Bio-Rad Laboratories, Hercules, Calif.).
Gel mobility shift assays.
Cells were grown in supplemented Lee’s liquid medium (7) to late log phase and then lysed according to methods previously described (52). Cell extracts contained between 10 and 12 mg of protein per ml. The incubation medium contained the following constituents: 25 mM HEPES (pH 7.9), 50 mM NaCl, 1 mM EDTA, 0.05% Nonidet P-40, 1 mM dithiothreitol, 5% glycerol, 5 μg of poly(dG-dC), and 7.5 μg of cellular extract, with or without 5 mM MgCl2. Approximately 12 μl of this mixture was incubated for 10 min at 25°C with or without unlabeled competitor DNA, as noted in Results. Fifty picograms of 32P-end-labeled DNA was then added, and the mixture was incubated for an additional 30 min at 25°C. Four microliters of a solution containing 25 mM HEPES, 50 mM NaCl, 1 mM EDTA, 0.01% xylene cyanol, and 0.01% bromophenol blue was added to each tube, and the tubes were then placed on ice prior to loading. Mixtures were loaded on a 3, 4, or 7% nondenaturing gel (39:1 acrylamide-to-bisacrylamide ratio in buffer containing 22.3 mM Tris-HCl [pH 8.1], 22.3 mM boric acid, 1 mM EDTA) which had been prerun for 1 h at 110 V. Gel electrophoresis was performed at a constant 100 V at 4°C for 6 h. Gels were transferred to Whatman 3MM paper, dried, and autoradiographed.
Oligonucleotide pairs M4 and M4A, M1 and M1A, and M5 and M5A (Table 1) were annealed by being boiled for 10 min in Sequenase buffer (U.S. Biochemicals, Cleveland, Ohio), followed by slow cooling to room temperature. Double-stranded oligonucleotides were purified on a 10% polyacrylamide gel (39:1) followed by elution in a solution containing 10 mM Tris HCl (pH 7.5) and 1 mM EDTA.
RESULTS
The white-opaque transition and OP4 expression.
Red 3/6, the ade2 auxotrophic derivative of strain WO-1 used in this study, underwent the white-opaque transition (Fig. 2A) at spontaneous frequencies comparable to those of parental strain WO-1 (3, 43, 44). OP4 was previously demonstrated to be transcribed exclusively in the opaque phase of the parental strain WO-1 (31), and that was also true for the ade2 derivative Red 3/6 (Fig. 2B).
FIG. 2.
The white-opaque transition of the adenine auxotroph Red 3/6 of C. albicans WO-1 and differential expression of OP4 in the opaque phase. (A) Cells of strain Red 3/6 switch spontaneously and reversibly at 25°C at frequencies of approximately 10−3 between a white- and an opaque-colony-forming phenotype. This reversible transition is accompanied by a distinct change in cellular morphology. White-phase cells are round while opaque-phase cells are twice as large, on average, and elongated and contain a large vacuole. (B) Opaque-phase cells of strain Red 3/6 differentially express the gene OP4. Northern blots of white- and opaque-phase cells were probed with OP4 and the constitutively expressed gene ADE2. Even though Red 3/6 is an adenine auxotroph, the mutant ade2 gene is constitutively expressed. Bar, 2 μm.
The upstream region of OP4.
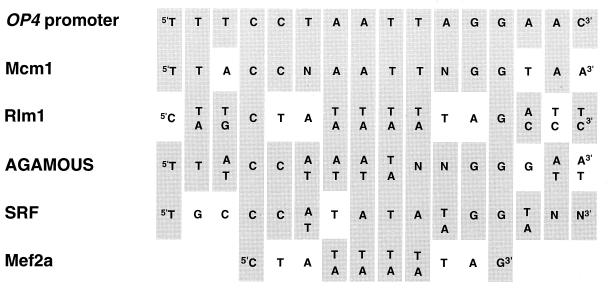
The 854-bp region upstream of the OP4 open reading frame contained several consensus sequences of elements which play _cis_-acting roles in the regulation of transcription in other systems (Fig. 3). Between −533 and −520 bp, there was a C-rich region (C box) (Fig. 3) that is also present in the upstream region of the opaque-phase-specific secreted aspartyl proteinase gene PEP1 (47), also referred to as SAP1 (57). Two TATA box consensus sequences (10) were present at −329 bp and at −187 bp (Fig. 3). A MADS box consensus binding sequence (33) was present between bp −416 and −403 and represented a perfect palindrome (Fig. 3). The sequence of this MADS box consensus binding site showed greater homology to the Mcm1 binding site of S. cerevisiae (1) than to other MADS box consensus sequences, including the Rlm1 binding site of S. cerevisiae (13), the serum response factor (SRF) binding site of humans (35), the Mef2a binding site of humans (33), and the more degenerate AGAMOUS binding site of plants (17) (Fig. 4). Two PRE consensus sequences (23) were present at −559 and −454 bp (Fig. 3). In S. cerevisiae, PRE sequences are binding sites for Ste12 (14, 16). Ste12 and Mcm1 have been shown to interact in the regulation of transcription in S. cerevisiae (19, 34, 41).
FIG. 3.
The 854-bp sequence immediately upstream of the translational start site of opaque-phase-specific gene OP4. PRE, consensus sequences of binding sites for Ste12 of S. cerevisiae (14, 16, 23); C-box, a C-rich sequence also identified in the upstream region of the coordinately activated, opaque-phase-specific gene PEP1 (SAP1) (30); MADS box binding site, MADS box consensus binding site homologous with the Mcm1 binding site of S. cerevisiae (2); TATA, TATA box consensus sequence (10); tsp, transcription start point.
FIG. 4.
Homology between the MADS box consensus binding site in the OP4 promoter and MADS box consensus binding site of S. cerevisiae, humans, and plants. Shown are Mcm1 binding site of S. cerevisiae (1), Rlm1 binding site of S. cerevisiae (13), AGAMOUS binding site of Arabidopsis (17), SRF binding site of humans (35), and Mef2a binding site of humans (33). Grey boxes denote conserved nucleotides.
Functional characterization of deletion derivatives of the OP4 promoter.
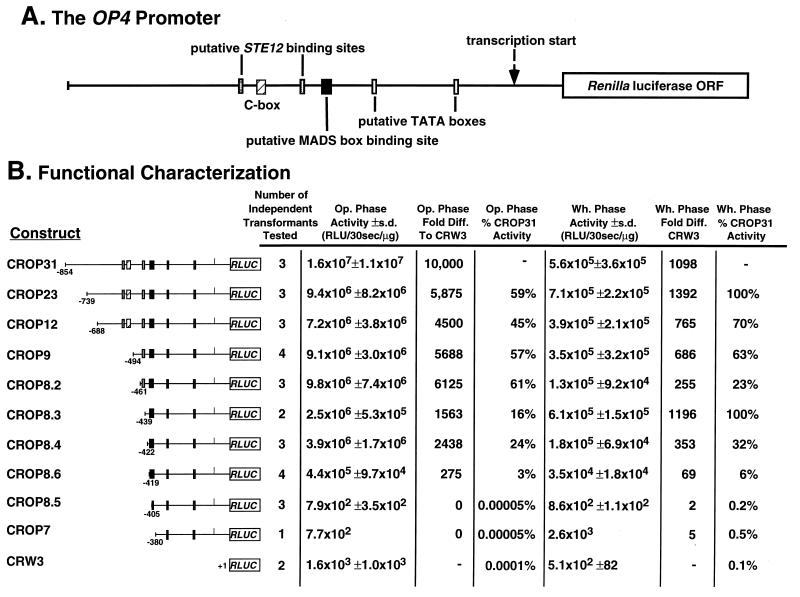
The promoter of OP4, therefore, contains several putative _cis_-acting sequences that have been shown to play _cis_-acting regulatory roles in S. cerevisiae as well as in higher eukaryotes. However, if any of these or other promoter sequences in fact play regulatory roles in the phase-specific transcription of OP4, it was essential first to functionally characterize the promoter through the analysis of deletion derivatives (52, 55). The plasmid pCROP31 contains the 854-nucleotide untranslated upstream region of OP4 fused to the RLUC open reading frame (Fig. 5A) and was previously demonstrated to regulate luciferase expression in an accurate phase-specific manner during the white-opaque transition when integrated at the ectopic ade2 locus in Red 3/6 (54). pCROP31 and the deletion derivatives pCROP23, pCROP12, pCROP9, pCROP8.2, pCROP8.3, pCROP8.4, pCROP8.6, pCROP8.5, pCROP7, and pCRW3 (Fig. 5B) were each linearized at the ADE2 locus and then used to transform the ade2 derivative strain Red 3/6. In each case, 10 independent transformants were chosen for Southern analysis to verify site-specific integration at the ade2 locus of strain Red 3/6. All transformants contained bands at 20.0 and 11.0 kb, which represented expected fragments containing the endogenous ade2 gene of Red 3/6. In addition, all transformants contained a 4.6-kb band which represented the plasmid-derived E fragment of the plasmid (54). pCROP31, pCROP8.2, pCROP8.3, pCROP8.4, and pCROP8.5 transformants contained a 1.2-kb band, which did not contain OP4 promoter sequences because of the presence of an extra _Bam_HI site not deleted during cloning of the promoter (data not shown). The pCROP23 transformant contained a fragment that was 2.7 kb. This fragment became progressively smaller in the subsequent deletion constructs pCROP12, pCROP10, and pCROP9 since it contained the sequentially smaller OP4 promoter sequence (Fig. 5B). In the case of these latter constructs, the plasmids were cloned as _Pst_I/_Sma_I fragments and the _Bam_HI site was not deleted during cloning. The 11- and 20-kb bands containing the endogenous ade2 locus of Red 3/6 were approximately equal in intensity in all individual transformants and in untransformed Red 3/6. However, the plasmid bands of transformant clones of a particular construct differed in intensity, indicating differences in the number of plasmid inserts. Since _Bam_HI sites flanking the integration site were involved in the genesis of fragments with variable intensity in the Southern blots, and since there were no variations in the size of fragments between independent clones of the same construct, differences in the intensity of fragments encompassing the plasmid must represent differences in the number of tandem repeats at the ade2 locus of Red 3/6. This conclusion was supported by the observation that Rluc activity correlated with the varying intensity of inserts in transformants in which RLUC was activated (data not shown). We, therefore, selected 1 to 4 of the 10 transformants of each construct with bands at 4.6 and either 2.7 or 1.2 kb that had intensities reflecting a single copy of the plasmid insert. Unfortunately, none of the 10 transformants of pCROP10 had band intensities consistent with a single insert, so a pCROP10 transformant was not included in the functional analysis of the OP4 promoter.
FIG. 5.
Functional characterization of the OP4 promoter through a set of deletion derivatives. (A) Model of the OP4 promoter. Sequences homologous to known regulatory sequences in the promoters of other genes as well as the C box also found in the promoter of the coordinately regulated PEP1 (SAP1) gene are noted. The sequence of the promoter is presented in Fig. 3. ORF, open reading frame. (B) Activity of Rluc under the regulation of the deletion derivatives of the OP4 promoter in the opaque and white phases of switching. s.d., standard deviation; Op., opaque; Wh., white; Diff., difference.
In Fig. 5A, a model of the OP4 promoter is presented in which landmark sequences are identified. In Fig. 5B, Rluc activities in the white and opaque phases are presented for the series of deletion constructs. Rluc activity in the opaque phase of the transformant harboring CROP31, which contained the entire 854-bp upstream region of OP4, was considered maximum expression. Deletion of the first 115 bp of the promoter region, between −854 and −739 bp in CROP23, resulted in a reduction of approximately 40% of Rluc activity in the opaque phase (Fig. 5B). Deletion of the next 51 bp, between −739 and −688 bp in CROP12, and subsequent deletions of the next 194 bp, between −688 and −494 bp in CROP9, and between −494 and −461 bp in CROP8.2, which removed the first Ste12 binding site consensus sequence and C box, resulted in no additional decrease in Rluc activity in the opaque phase (Fig. 5B). Sequential deletions of the next 22 and 18 bp in CROP8.3 and CROP8.4, respectively, which removed the second Ste12 binding site consensus sequence, resulted in a further reduction of 45% of Rluc activity in the opaque phase (Fig. 5B). Although the cumulative deletions between −854 and −422 bp reduced activity by approximately 80%, the remaining 20% was still approximately 1,600 times the level measured in the promoterless construct CRW3 (Fig. 5B). Deletion of the next 3 bp to nucleotide −419 in CROP8.6 resulted in a further decrease of approximately 20% in activity, but this level of activity was still 275 times that of the promoterless construct CRW3 (Fig. 5B). Deletion of the next 15 bp, between −419 and −404 bp in CROP8.5, however, resulted in the nearly complete loss of Rluc activity in the opaque phase, an activity change from 4.4 × 105 ± 9.7 × 104 to 7.9 × 102 ± 3.5 × 102 RLUs (Fig. 5B). This deletion removed all but the most proximal base pairs of the MADS box consensus binding site (Fig. 3). Subsequent deletion of the next 24 bp, between −404 and −380 bp in CROP7, which removed the rest of the MADS box consensus binding site, had no additional effect on the level of Rluc activity in the opaque phase, and complete deletion of the OP4 promoter (CRW3) resulted in Rluc activity similar to that of CROP8.5 and CROP7. These results suggest that a region beginning between −419 and −404 bp contains a transcription activation sequence. This region coincides with the palindromic MADS box consensus binding site. These results also suggest that regions upstream of −419 bp play a role in activation and that the region between −404 bp and the transcription start site has no intact activation sequence(s).
OP4 expression in the white phase of the white-opaque transition is undetectable by Northern analysis in either strain WO-1 (31) or the ade2 derivative Red 3/6 (Fig. 2B). The activity of Rluc in the white phase of the transformant harboring CROP31, which contains the entire 854-bp promoter region of OP4 upstream of RLUC, was 1/29 that in the opaque phase. Because each population of white-phase cells can contain up to a few percent opaque cells resulting from spontaneous switching (33, 43–45), it is not clear what portion of white-phase activity is due to a basal level of white-phase expression and what portion is due to the unpredictable levels of opaque-phase cell contamination. Deletions between −854 and −422 bp resulted in a general decline in activity in the white phase to roughly 30% of the activity of the full-length promoter construct (Fig. 5B). However, a deletion between −422 and −419 bp in CROP8.6 decreased activity to 6% that of the full promoter, and a deletion between −419 and −404 bp in CROP8.5, the region containing the upstream portion of the MADS box consensus binding site, resulted in the complete loss of the Rluc activity (Fig. 5B). Subsequent deletions, including full-length promoter deletion (CRW3), had no additional effect on Rluc activity (Fig. 5B). The complete loss of Rluc activity after deletion of the MADS box consensus binding site was, therefore, similar in both white- and opaque-phase cells.
Mutations in the C. albicans MADS box consensus binding site diminish OP4 transcription.
In order to demonstrate that an intact MADS box consensus binding site is necessary for OP4 transcription, a number of point mutations of the consensus binding site were generated in CROP8.6, which is a deletion derivative beginning at nucleotide −419, 3 bp upstream from the MADS box consensus sequence. CROP8.6 supported transcription at a level 133-fold higher than that of the promoterless construct CRW3 (4.4 × 105 ± 9.7 × 104 versus 1.6 × 103 ± 1.0 × 103 RLUs) but 9-fold lower than that of CROP8.4 (4.4 × 105 versus 3.9 × 106 RLUs) (Fig. 5B). A series of palindromic point mutations were generated that were based on those changes in the Mcm1 binding site of S. cerevisiae that resulted in loss of function (1). These mutations included one with nucleotide transitions (CRM11) as well as several with nucleotide transversions (CRM7, CRM8, CRM9, and CRM10) (Table 2). The majority of point mutations resulted in activities between 0.8 and 2% that of CROP8.6, which contained the unmodified MADS box consensus binding site (Table 2). All of these mutant constructs retained palindromic sequences. In two of the generated mutants, mammalian MADS box sequences were substituted for the C. albicans MADS box consensus sequence. In mutant CRM1, the SRF consensus binding site sequence was substituted, and in CRM3, the MEF2A consensus binding site sequence was substituted (33). Both contained intact palindromes, but only CRM3 exhibited significant activity (Table 2).
TABLE 2.
Functional characterization of the OP4 MADS box consensus sequence through a set of mutations including transitions and transversionsa
| Clone | Sequence between −419 and −400 of the OP4 promoter and in mutants | No. of individual transformants tested | Opaque-phase value | ||
|---|---|---|---|---|---|
| Activity (± SD) (RLU/30 s/μg) | % CROP8.6 activity | Fold difference from CrW3 | |||
| CROP8.6 | AATTTCCTAATTAGGAACTG | 4 | 4.4 × 105 ± 9.7 × 104 | 133 | |
| CRM7 | AATGTCCTAATTAGGACCTG | 3 | 3.6 × 103 ± 1.6 × 103 | 0.8 | 1 |
| CRM8 | AATTTGCTAATTAGCAACTG | 3 | 5.4 × 103 ± 1.4 × 103 | 1.2 | 2 |
| CRM9 | AATTTCCTACGTAGGAACTG | 3 | 5.7 × 103 ± 3.9 × 102 | 1.3 | 2 |
| CRM10 | AATTTCGTAATTACGAACTG | 4 | 4.9 × 103 ± 3.7 × 103 | 1.1 | 1 |
| CRM11 | AATTCCCTAATTAGGGAGTG | 3 | 9.2 × 103 ± 4.1 × 103 | 2.1 | 3 |
| CRM1 (SRF) | -ATTTCCATATATGGATCTG | 3 | 4.2 × 103 ± 2.8 × 103 | 1.0 | 1 |
| CRM3 (MEF2A) | AATTTCTATTTATAGAACTG | 5 | 8.1 × 104 ± 2.5 × 104 | 18.0 | 25 |
| CRM5 (PEP1) | AATTTCCATACTTCTAACTC | 3 | 3.7 × 103 ± 5.8 × 102 | 0.8 | 1 |
| CRM6 (WH11) | AATTTCCTACAATAGAACTG | 3 | 3.3 × 103 ± 1.7 × 103 | 0.8 |
In the last two mutant constructs, MADS box-like sequences identified in the promoters of two additional phase-regulated genes, the opaque-phase-specific gene PEP1 (SAP1) (31, 47) and the white-phase-specific gene WH11 (51, 52, 55), were substituted for the OP4 MADS box consensus binding site. In construct CRM5, nucleotides −418 to −405 of OP4 were substituted with nucleotides −557 to −544 of PEP1 (SAP1) (47), and in construct CRM6, nucleotides −417 to −405 of OP4 were substituted with nucleotides −128 to −115 of WH11 (52, 55). Neither of these mutations activated transcription in the opaque phase, leading to the conclusion that activation of OP4 transcription is regulated by _cis_-acting sequences different from those regulating the other opaque-phase-specific gene PEP1 and the white-phase-specific gene WH11.
The Mcm1 consensus binding region is necessary for transcription of the OP4 gene.
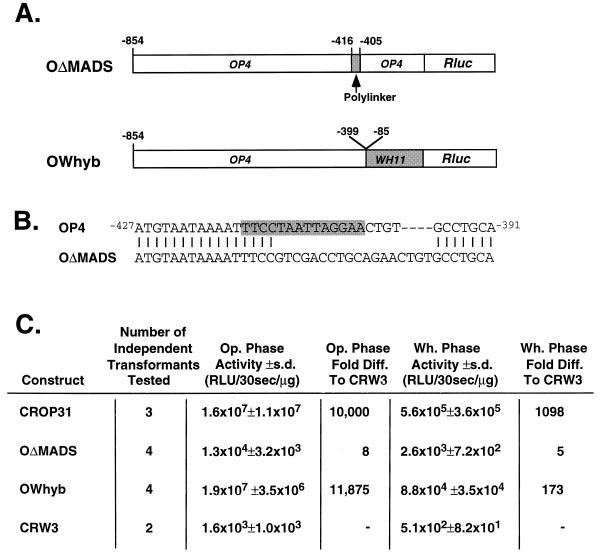
Nested 5′ deletions suggested that there were sequences upstream of the OP4 MADS box consensus binding site that enhanced the level of MADS box-activated OP4 transcription, but these deletions did not represent direct tests for the presence of redundant sites in the upstream region that functioned in the absence of the MADS box. To test for redundant sites, a construct was made in which the nucleotide sequence between −416 and −405 of the 854 nucleotides of the OP4 promoter, which contained two-thirds of the downstream portion of the MADS box, was replaced with polylinker in the construct OΔMADS (Fig. 6A and B). OΔMADS did not support transcription. The level of Rluc activity in the transformant harboring OΔMADS upstream of RLUC was only slightly higher (eightfold) than that in the transformant harboring the promoterless construct CRW3 (Fig. 6C). This result demonstrates that no transcription activation sequences reside upstream of the MADS box consensus binding site that can activate gene transcription in a phase-specific manner.
FIG. 6.
Functional characterization of the OP4 MADS box consensus sequence through substitution of consensus sequence with polylinker (OΔMADS) and replacement of the promoter downstream of the consensus sequence with the downstream region of the WH11 promoter (OWhyb). (A) Models of the two constructs OΔMADS and OWhyb. (B) Sequence of the substitution in OΔMADS. The OP4 activation sequence is highlighted. (C) Activity of Rluc under the regulation constructs OΔMADS and OWhyb. Note that CROP31 contain the complete OP4 promoter and that CRW3 is a promoterless construct. Op., opaque; Wh., white; s.d., standard deviation; Diff., difference.
In a final construct, the OP4 activation sequence was placed upstream of the transcriptional start region of the WH11 promoter, which in turn was placed upstream of RLUC in construct OWhyb (Fig. 6A). Transformants containing the parental plasmid pCRW5ΔAf were previously shown to have no luciferase activity in either the white or the opaque phase (55). In OWhyb, the nucleotide sequence of OP4 between −854 and −399, which contains the MADS box consensus binding site, was fused to the nucleotide sequence of WH11 between −85 and −1 (52, 55), which contains the transcription start site and TATA binding region of the WH11 promoter, but not the two transcription activation domains. This construct tested whether any sequence proximal to the MADS box consensus binding sequence was necessary for activation at the latter site. The level of Rluc activity in the transformant harboring OWhyb in the opaque phase was similar to that of pCROP31, which harbors the entire OP4 promoter (Fig. 6C). In the white phase of this transformant, there was no Rluc activity (Fig. 6C), demonstrating that the OP4 activation sequence is sufficient for opaque-phase-specific activation. These data confirm that the C. albicans MADS box consensus binding site is both necessary and sufficient to promote opaque-phase transcription, even with chimeric downstream promoter sequences.
Complex formation between the OP4 promoter and cell protein extract components.
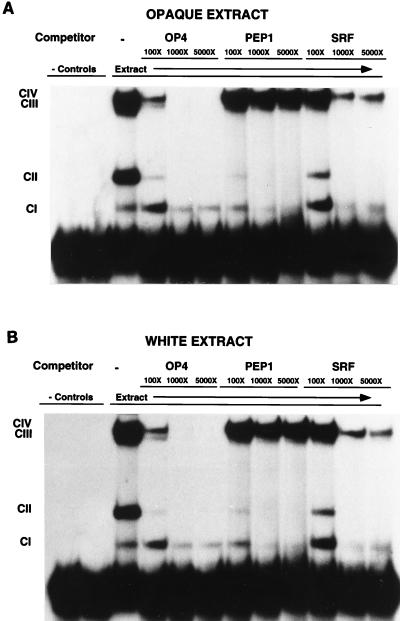
Gel mobility shift assays were performed with an oligonucleotide containing the 14-bp MADS box consensus binding site sequence of the OP4 promoter flanked by the 16 upstream and 8 downstream nucleotides. When opaque-phase cell extract was used in the assay, the four complexes CI, CII, CIII, and CIV formed (lane 3, Fig. 7A). No complexes formed in a negative control in which no protein extract was added (lane 1, Fig. 7A), and no complexes formed in a negative control in which bovine serum albumin was added in place of protein extract (lane 2, Fig. 7A). When the same oligonucleotide in an unlabeled form was used at 100× concentration as a competitor of binding, formation of three of the radiolabeled complexes, CII, CIII, and CIV, was reduced (lane 4, Fig. 7A), and when the oligonucleotide was used as a competitor at 1,000× and 5,000×, formation of these latter three radiolabeled complexes was undetectable (lanes 5 and 6, Fig. 7A). These results demonstrate that complexes CII, CIII, and CIV formed as a result of interactions between the oligonucleotide containing the MADS box consensus binding site and components of opaque-phase cell extract. When similar gel shift assays were performed with white- rather than opaque-phase cell extract, virtually identical results were obtained (Fig. 7B), demonstrating that no detectable phase-specific complexes formed with the 38-bp oligonucleotide containing the OP4 transcription activation sequence.
FIG. 7.
Gel mobility shift assays with an oligonucleotide containing the MADS box consensus binding site flanked by 16 upstream and 8 downstream nucleotides of the OP4 promoter and either white- (A) or opaque-phase (B) cell extract. Lanes 1, no protein extract; lanes 2, bovine serum albumin in place of protein extract; lanes 3, white- or opaque-phase protein extract; lanes 4, 5, and 6, increasing concentrations of unlabeled oligonucleotide containing the putative MADS box consensus binding site of OP4; lanes 7, 8, and 9, increasing concentrations of unlabeled oligonucleotides containing the putative MADS box consensus binding site of PEP1 (SAP1); lanes 10, 11, and 12, increasing concentrations of unlabeled oligonucleotide containing the SRF consensus binding site. Double-stranded oligonucleotides used in the gel mobility shift experiments include OP4 (M4 and M4A in Fig. 1), the 38 bp of the OP4 promoter containing the MADS box consensus binding site; PEP1 (SAP1) (M5 and M5A in Fig. 1), the 38 bp of the OP4 promoter in which the OP4 MADS box consensus binding site is replaced with the putative MADS box consensus binding site in the promoter of the gene PEP1 (SAP1) (30), which is coordinately regulated with OP4 (31); and SRF (M1 and M1A), the 38 bp of the OP4 promoter in which the OP4 MADS box consensus binding site is replaced with the SRF binding site of humans (35). Lower exposures discriminated the two bands CIV and CIII in the lanes containing no competitor, OP4 competitor, and 100× SRF competitor (data not shown).
PEP1 (SAP1) is coordinately regulated with OP4 in the white-opaque-phase transition (31). To test whether the putative MADS box consensus binding site in the OP4 promoter region plays a role in the formation of complexes CII, CIII, and CIV, a sequence in the PEP1 (SAP1) promoter region with homology to the OP4 MADS box consensus binding site was substituted for the homologous portion in the oligonucleotide used as a competitor in the gel mobility assay. The _PEP1 (SAP1)_-derived oligonucleotide competed for complex CII but not for complexes CIII or CIV (lanes 7, 8, and 9, Fig. 7), suggesting that formation of the CII complex is not affected by the nucleotide differences between the OP4 and PEP1 (SAP1) MADS box consensus binding sites, while formation of the CIII and CIV complexes is.
In a similar fashion, the binding site for SRF (35), which is homologous to the greater portion of the OP4 MADS box consensus binding site, was replaced in the oligonucleotide used as a competitor in the gel mobility shift assay. The SRF-derived oligonucleotide competed for complexes CII and CIII but not CIV (lanes 10, 11, and 12, Fig. 7), suggesting that formation of the CII and CIII complexes is not affected by the nucleotide differences between the OP4 and SRF MADS box consensus binding sites, while formation of the CIV complex is.
DISCUSSION
Switching in C. albicans and related species occurs both in commensal and in infecting populations and affects a majority of putative virulence characteristics (4, 6, 18, 21, 22, 45, 46, 57). In the case of C. albicans WO-1, switching between the white and the opaque phases is accompanied by the differential expression of phase-specific genes (48, 49). In the white phase, cells express the white-phase-specific gene WH11 (51), and in the opaque phase, cells express opaque-phase-specific genes, including OP4 (31), PEP1 (SAP1) (30), SAP3 (57), and CDR3 (6). Although the molecular basis of the fundamental switch event in C. albicans has not been established (48, 49), it seems likely that the coordinated activation and deactivation of so many genes in the switching process involve the phase-specific regulation of _trans_-acting factors, especially since the phase-specific genes initially analyzed have been demonstrated to be unlinked (31, 51).
The first detailed characterization of a promoter of a C. albicans gene was that of the white-phase-specific gene WH11 (52). Through the functional analysis of deletion derivatives of the WH11 promoter, it was demonstrated that there were two strong and one relatively weak transcription activation sequence and that the two strong activation sequences functioned synergistically (52, 55). The results of this analysis suggested no repressor sequences and led to the conclusion that WH11 was regulated solely through activation. Gel mobility experiments using the WH11 promoter and white- or opaque-phase cell extracts identified two white-phase-specific complexes for each of the strong activation sequences but no opaque-phase-specific complexes, leading to the tentative conclusion that white-phase-specific transcription factors interact with the strong transcription activation sequences to initiate WH11 transcription in a phase-specific manner (52, 55). Although the upstream region of WH11 contained a number of sequences homologous to the regulatory sequences of genes in other organisms, none of them fell within the transcription activation sequences determined by deletion analysis, leading to the conclusion that the transcription activation sequences for WH11 were unique (52).
Here, we have undertaken a similar functional characterization of the promoter of the opaque-phase-specific gene OP4. OP4 is of particular interest for several reasons. First, it is differentially expressed not only in the opaque phase of strain WO-1 (31) but also in the variant phenotypes of the switching repertoire of the laboratory strain 3153A (32). Second, misexpression of OP4 in the white phase may affect virulence (27). Here, we have found that one major activation sequence is essential for OP4 transcription in the opaque phase and that sequences upstream of this major transcription activation sequence enhance activation at this site. The major activation sequence is located within nucleotides −422 and −404. Deletion of the OP4 promoter between nucleotides −854 and −404 or partial replacements of the activation sequence with polylinker between nucleotides −416 and −405 result in the complete loss of activation. These results demonstrate that the sequences flanking the activation site both upstream and downstream are insufficient for activation in the opaque phase.
Perhaps the most intriguing result of this study lies in the sequence of the 18-bp activation domain of the OP4 promoter. This region includes a palindromic 14-bp MADS box consensus binding site. Of the MADS box consensus binding sites so far identified, the sequence most closely related to that of C. albicans is the binding site of Mcm1 in S. cerevisiae (Fig. 4). In S. cerevisiae, Mcm1 binds to MADS box binding sites as a dimer (9, 11, 41). Mcm1 is a global regulator, interacting combinatorially with other proteins to either activate or repress the transcription of a number of genes (24). Mcm1 interacts with MATα1 to activate genes (15, 50), with MATα2 to repress genes (15, 50), and with Ste12 to activate genes (15, 34, 50) in the mating process and cell cycle of S. cerevisiae. It also interacts with Dff to activate genes in the cell cycle (28). The correlation between the major transcription activation site of the OP4 promoter and the conserved MADS box consensus binding sequence of S. cerevisiae (1) suggests that some aspects of phenotypic switching involve regulation by an Mcm1-like factor in C. albicans, a possibility now being actively pursued.
In a functional analysis of the Mcm1 binding site of an Mcm1/α2-activated promoter of S. cerevisiae, Acton et al. (1) demonstrated by point mutation that specific nucleotides in the site were necessary for activation. We engineered similar point mutations in the MADS box consensus sequence of the OP4 promoter, and as in the study by Acton et al. (1), all of the mutations were engineered so that the characteristic palindrome was maintained. Mutations were generated at five specific nucleotide positions within the consensus site. All five mutations led to loss of function. These results were similar to those obtained by Acton et al. (1) for the Mcm1 binding site with one notable exception. When an A-to-C transition was made at the second nucleotide of the Mcm1 site, there was only a twofold reduction in activation. In contrast, the same transition mutation in the OP4 MADS box consensus site resulted in the complete elimination of activation. Acton et al. (1) also found that this latter mutation resulted in a 10-fold reduction in repression. While we cannot measure repression in the white phase at the MADS box consensus binding site of the OP4 promoter, we have found no increase in activation that would indicate a reduction in repression in any of the mutations that we generated in the consensus sequence.
When the MADS box consensus binding site sequence was replaced with the human MADS box sequence SRF (35) or MEF2A (33), there was no activation in the former case and low, but significant, activation in the latter case. Since OP4 is coordinately regulated with the opaque-phase-specific gene PEP1 (SAP1) (31) and the white-phase-specific gene WH11 (51), we also replaced the OP4 MADS box consensus sequence with homologous sequences in the PEP1 (SAP1) and WH11 promoters. In neither case did we obtain activation.
To test whether phase-specific protein-DNA interactions occur at the 18-bp activation site of the OP4 promoter in a fashion similar to that demonstrated for the two activation sequences DAS and PAS of the promoter of the white-phase-specific gene WH11 (52), gel mobility shift assays were performed with white- or opaque-phase cell extracts. Three specific DNA-protein complexes, CII through CIV, which were verified by competition experiments formed. Two of the binding complexes, CII and CIII, could each be competed by one or both of two oligonucleotides with altered MADS box consensus binding sites. Complex CIV, however, could not be competed with constructs containing altered MADS box consensus binding sites, suggesting that there was a stringent requirement for the exact OP4 MADS box consensus binding site sequence in the interaction generating CIV complexes. Unlike the WH11 activation domains, none of the three complexes were opaque phase specific under the conditions employed, and altered magnesium levels did not affect formation of any of the complexes (27a). If the complexes represent interactions between the MADS box consensus binding site and an Mcm1-type protein, this result would not be surprising since regulation, in this case, might be through combinatorial protein-protein interactions (56) or phosphorylation of the Mcm1 protein (25), neither of which can be as readily assayed by the gel mobility shift assays used in this study.
Recently, Braun and Johnson (8) demonstrated that disruption of TUP1 results in exclusive growth of C. albicans in the pseudohyphal rather than the yeast form. This has led to a model in which Tup1 functions as a repressor of genes necessary for pseudohyphal growth, which when knocked out results in constitutive pseudohyphal growth. In S. cerevisiae, Tup1 and Ssn6 are necessary for MATα2 and Mcm1 to function in the repression of transcription (20). A similar combinatorial interaction may be involved in the repression of OP4 transcription in the white phase of C. albicans WO-1. Indeed, there are several parallels between the opaque phase and hypha formation (45), including the inactivation of WH11 transcription (51). The possible roles of Mcm1 and Tup1 in the regulation of OP4 at the MADS box consensus binding site are now under intensive investigation.
ACKNOWLEDGMENTS
We are grateful to Deborah Wessels for help in photographing living cells.
This work was supported by Public Health Service grants AI39735 and DE10758 from the National Institutes of Health. S.R.L. was supported by training grant AG00214 from the National Institutes of Health.
REFERENCES
- 1.Acton T, Zhong H, Vershon A. DNA-binding specificity of Mcm1: operator mutations that alter DNA-bending and transcriptional activities by a MADS box protein. Mol Cell Biol. 1997;17:1881–1889. doi: 10.1128/mcb.17.4.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammerer G. Identification, purification, and cloning of a polypeptide (PRTF/GRM) that binds to mating-specific promoter elements in yeast. Genes Dev. 1990;4:299–312. doi: 10.1101/gad.4.2.299. [DOI] [PubMed] [Google Scholar]
- 3.Anderson J, Soll D. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987;169:5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson J, Cundiff L, Schnars B, Gao M, Mackenzie I, Soll D. Hypha formation in the white-opaque transition of Candida albicans. Infect Immun. 1989;57:458–467. doi: 10.1128/iai.57.2.458-467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson J, Mihalik R, Soll D. Ultrastructure and antigenicity of the unique cell wall pimple of the Candida opaque phenotype. J Bacteriol. 1990;172:224–235. doi: 10.1128/jb.172.1.224-235.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balan I, Alareo A, Raymond M. The Candida albicans CDR3 gene codes for an opaque-phase ABC transporter. J Bacteriol. 1997;179:7210–7218. doi: 10.1128/jb.179.23.7210-7218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedell G, Soll D. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun. 1979;26:348–354. doi: 10.1128/iai.26.1.348-354.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun B, Johnson A. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 9.Bruhn L, Hwang S, Sprague G. The N-terminal 96 residues of MCM1, a regulator of cell type-specific genes in Saccharomyces cerevisiae, are sufficient for DNA binding, transcription activation, and interaction with α1. Mol Cell Biol. 1992;12:3563–3572. doi: 10.1128/mcb.12.8.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavallini B, Huet J, Plassat J, Sentenac A, Egly J, Chambon P. A yeast activity can substitute for the HeLa cell TATA box factor. Nature. 1988;334:77–80. doi: 10.1038/334077a0. [DOI] [PubMed] [Google Scholar]
- 11.Christ C, Tye B. Functional domains of the yeast transcription/replication factor MCM1. Genes Dev. 1991;5:751–763. doi: 10.1101/gad.5.5.751. [DOI] [PubMed] [Google Scholar]
- 12.Church G M, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodou E, Treisman R. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol Cell Biol. 1997;17:1848–1859. doi: 10.1128/mcb.17.4.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolan J, Kirkman C, Fields S. The yeast Ste12 protein binds to the DNA sequence mediating pheromone induction. Proc Natl Acad Sci USA. 1989;86:5703–5707. doi: 10.1073/pnas.86.15.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolan J, Fields S. Cell-type-specific transcription in yeast. Biochim Biophys Acta. 1991;1088:155–169. doi: 10.1016/0167-4781(91)90051-m. [DOI] [PubMed] [Google Scholar]
- 16.Errede B, Ammerer G. STE12, a protein involved in cell-type-specific transcription and signal transduction in yeast, is part of protein-DNA complexes. Genes Dev. 1989;3:1349–1361. doi: 10.1101/gad.3.9.1349. [DOI] [PubMed] [Google Scholar]
- 17.Huang H, Mizukami Y, Hu Y, Ma H. Isolation and characterization of the binding sequences for the product of the Arabidopsis floral homeotic gene AGAMOUS. Nucleic Acids Res. 1993;21:4769–4776. doi: 10.1093/nar/21.20.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hube B, Monod M, Schofield D, Brown A, Gow N. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol Microbiol. 1994;14:87–99. doi: 10.1111/j.1365-2958.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 19.Hwang-Shum J-J, Hagen D, Jarvis E, Westby C, Sprague G. Relative contributions of MCM1 and STE12 to transcriptional activation of a- and α-specific genes from Saccharomyces cerevisiae. Mol Gen Genet. 1991;227:197–204. doi: 10.1007/BF00259671. [DOI] [PubMed] [Google Scholar]
- 20.Keleher C, Redd M, Schultz J, Carlson M, Johnson A. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell. 1992;68:709–719. doi: 10.1016/0092-8674(92)90146-4. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy M, Rogers A, Hanselmen L, Soll D, Yancey R. Variation in adhesion and cell surface hydrophobicity in Candida albicans white and opaque phenotypes. Mycopathologia. 1988;102:149–156. doi: 10.1007/BF00437397. [DOI] [PubMed] [Google Scholar]
- 22.Kolotila M, Diamond R. Effects of neutrophils and in vitro oxidants on survival and phenotypic switching of Candida albicans WO-1. Infect Immun. 1990;58:1174–1179. doi: 10.1128/iai.58.5.1174-1179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kronstad J, Holly J A, MacKay V. A yeast operator overlaps an upstream activation site. Cell. 1987;50:369–377. doi: 10.1016/0092-8674(87)90491-0. [DOI] [PubMed] [Google Scholar]
- 24.Kuo M, Grayhack E. A library of yeast genomic MCM1 binding sites contains genes involved in cell cycle control, cell wall and membrane structure, and metabolism. Mol Cell Biol. 1994;14:348–359. doi: 10.1128/mcb.14.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo M, Nadeau E, Grayhack E. Multiple phosphorylated forms of the Saccharomyces cerevisiae Mcm1 protein include an isoform induced in response to high salt concentrations. Mol Cell Biol. 1997;17:819–832. doi: 10.1128/mcb.17.2.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kvaal C, Srikantha T, Soll D. Misexpression of the white phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect Immun. 1997;65:4468–4475. doi: 10.1128/iai.65.11.4468-4475.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kvaal, C., T. Srikantha, and D. R. Soll. Unpublished data.
- 27a.Lockhart, S., and D. R. Soll. Unpublished results.
- 28.Lydall D, Ammerer G, Nasmyth K. A new role for MCM1 in yeast: cell cycle regulation of SW15 transcription. Genes Dev. 1991;5:2405–2419. doi: 10.1101/gad.5.12b.2405. [DOI] [PubMed] [Google Scholar]
- 29.Matthews J, Hori K, Cormier M. Purification and properties of Renilla reniformis luciferase. Biochemistry. 1977;16:85–91. doi: 10.1021/bi00620a014. [DOI] [PubMed] [Google Scholar]
- 30.Morrow B, Srikantha T, Soll D. Transcription of the gene for a pepsinogen, PEP1, is regulated by white-opaque switching in Candida albicans. Mol Cell Biol. 1992;12:2997–3005. doi: 10.1128/mcb.12.7.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrow B, Srikantha T, Anderson J, Soll D. Coordinate regulation of two opaque-phase-specific genes during white-opaque switching in Candida albicans. Infect Immun. 1993;61:1823–1828. doi: 10.1128/iai.61.5.1823-1828.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrow B, Ramsey H, Soll D. Regulation of phase-specific genes in the more general switching system of Candida albicans strain 3153A. J Med Vet Mycol. 1994;32:287–294. doi: 10.1080/02681219480000361. [DOI] [PubMed] [Google Scholar]
- 33.Nurrish S, Treisman R. DNA binding specificity determinants in MADS-box transcription factors. Mol Cell Biol. 1995;15:4076–4085. doi: 10.1128/mcb.15.8.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oehlen L, McKinney J, Cross F. Ste12 and Mcm1 regulate cell cycle-dependent transcription of FAR1. Mol Cell Biol. 1996;16:2830–2837. doi: 10.1128/mcb.16.6.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollock R, Treisman R. A sensitive method for the determination of protein-DNA binding specificities. Nucleic Acids Res. 1990;18:6197–6204. doi: 10.1093/nar/18.21.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pomes R, Gil C, Nombela C. Genetic analysis of Candida albicans morphological mutants. J Gen Microbiol. 1985;131:2107–2113. doi: 10.1099/00221287-131-8-2107. [DOI] [PubMed] [Google Scholar]
- 37.Primig M, Winkler H, Ammerer G. The DNA binding and oligomerization domain of MCM1 is sufficient for its interaction with other regulatory proteins. EMBO J. 1991;10:4209–4218. doi: 10.1002/j.1460-2075.1991.tb04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rikkerink E, Magee B, Magee P. Opaque-white phenotype transition: a programmed morphological transition in Candida albicans. J Bacteriol. 1988;170:895–899. doi: 10.1128/jb.170.2.895-899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scherer S, Stevens D. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J Clin Microbiol. 1987;25:675–679. doi: 10.1128/jcm.25.4.675-679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiestl R, Geitz R. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 41.Sengupta P, Cochran B. The PRE and PQ box are functionally distinct yeast pheromone response elements. Mol Cell Biol. 1990;10:6809–6812. doi: 10.1128/mcb.10.12.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slutsky B, Buffo J, Soll D. High-frequency switching of colony morphology in Candida albicans. Science. 1985;230:666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- 43.Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll D. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J Bacteriol. 1987;169:189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soll D, Anderson J, Bergen M. The developmental biology of the white-opaque transition in Candida albicans. In: Prasad R, editor. The molecular biology of Candida albicans. Berlin, Germany: Springer-Verlag; 1991. pp. 20–45. [Google Scholar]
- 45.Soll D. High-frequency switching in Candida albicans. Clin Microbiol Rev. 1992;5:183–203. doi: 10.1128/cmr.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soll D. Switching and its possible role in Candida pathogenesis. In: Bennet J E, Hay R J, Peterson P K, editors. New strategies in fungal disease. Edinburgh, United Kingdom: Churchill Livingstone; 1992. pp. 156–172. [Google Scholar]
- 47.Soll, D., T. Srikantha, A. Chandrasekhar, B. Morrow, K. Schroeppel, and S. Lockhart. 1995. Gene regulation in the white-opaque transition of Candida albicans. Can. J. Bacteriol. 73(Suppl. 1):51049–51057.
- 48.Soll D. Gene regulation during high-frequency switching in Candida albicans. Microbiology. 1997;143:279–288. doi: 10.1099/00221287-143-2-279. [DOI] [PubMed] [Google Scholar]
- 49.Soll D. The emerging molecular biology of Candida albicans. ASM News. 1997;62:415–420. [Google Scholar]
- 50.Sprague G. Combinatorial associations of regulatory proteins and the control of cell type in yeast. Adv Genet. 1990;27:33–62. doi: 10.1016/s0065-2660(08)60023-1. [DOI] [PubMed] [Google Scholar]
- 51.Srikantha T, Soll D. A white-specific gene in the white-opaque switching system of Candida albicans. Gene. 1993;131:53–60. doi: 10.1016/0378-1119(93)90668-s. [DOI] [PubMed] [Google Scholar]
- 52.Srikantha T, Chandrasekhar A, Soll D. Functional analysis of the promoter of the phase-specific WH11 gene of Candida albicans. Mol Cell Biol. 1995;15:1797–1805. doi: 10.1128/mcb.15.3.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srikantha T, Morrow B, Schroppel K, Soll D. The frequency of integrative transformation at phase-specific genes of Candida albicans correlates with their transcriptional state. Mol Gen Genet. 1995;246:342–352. doi: 10.1007/BF00288607. [DOI] [PubMed] [Google Scholar]
- 54.Srikantha T, Klapach A, Lorenz W, Tsai L, Laughlin L, Gorman J, Soll D. The sea pansy Renilla reniformis luciferase serves as a sensitive bioluminescent reporter for differential gene expression in Candida albicans. J Bacteriol. 1996;178:121–129. doi: 10.1128/jb.178.1.121-129.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srikantha T, Tsai L, Soll D. The WH11 gene of Candida albicans is regulated in two distinct developmental programs through the same transcription activation sequences. J Bacteriol. 1997;179:3837–3844. doi: 10.1128/jb.179.12.3837-3844.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Treisman R, Ammerer G. The SRF and MCM1 transcription factors. Curr Opin Genet Dev. 1992;2:221–226. doi: 10.1016/s0959-437x(05)80277-1. [DOI] [PubMed] [Google Scholar]
- 57.White T, Miyasaki S, Agabian N. Three distinct secreted aspartyl proteinases in Candida albicans. J Bacteriol. 1993;175:6126–6133. doi: 10.1128/jb.175.19.6126-6133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]