L-amino acid sensing by the extracellular Ca2+-sensing receptor - PubMed (original) (raw)
L-amino acid sensing by the extracellular Ca2+-sensing receptor
A D Conigrave et al. Proc Natl Acad Sci U S A. 2000.
Abstract
The extracellular calcium (Ca(2+)(o))-sensing receptor (CaR) recognizes and responds to (i.e., "senses") Ca(2+)(o) as its principal physiological ligand. In the present studies, we document that the CaR is activated not only by extracellular calcium ions but also by amino acids, establishing its capacity to sense nutrients of two totally different classes. l-Amino acids, especially aromatic amino acids, including l-phenylalanine and l-tryptophan, stereoselectively mobilized Ca(2+) ions in the presence of the CaR agonists, Ca(2+)(o), gadolinium (Gd(3+)(o)), and spermine in fura-2-loaded human embryonic kidney (HEK-293) cells stably transfected with the human CaR. l-amino acid-dependent effects were observed above, but not below, a threshold level of Ca(2+)(o) of approximately 1.0 mM. l-Amino acids, particularly aromatic amino acids, also stereoselectively enhanced the sensitivity of the CaR to its agonists, Ca(2+)(o) and spermine. Branched-chain amino acids were almost inactive, and charged amino acids, including arginine and lysine, were much less effective than aromatic and other amino acids. l-amino acid mixtures emulating the amino acid composition of fasting human plasma reproduced the effects of high concentrations of individual l-amino acids on Ca(2+) mobilization and enhanced the sensitivity of the CaR to Ca(2+)(o). The data presented herein identify the CaR as a molecular target for aromatic and other l-amino acids. Thus, the CaR can integrate signals arising from distinct classes of nutrients: mineral ions and amino acids. The actions of l-amino acids on the CaR may provide explanations for several long recognized but poorly understood actions of dietary protein on calcium metabolism.
Figures
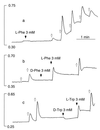
Figure 1
Effects of extracellular Ca2+,
l
-Phe, and
l
-Trp on Ca2+ mobilization in HEK-293 cells stably expressing the human CaR. HEK-5001 cells growing on glass coverslips were loaded with fura-2 and exposed to test agents in a stirred fluorescence cuvette at 37°C in the light path of a fluorometer. In all three panels, baseline Ca2+o was 0.5 mM, and 1-mM increments of Ca2+o were applied at the open arrows. The vertical bars indicate the fluorescence ratios (340/380). (a) Lack of effect of 3 mM
l
-Phe (closed arrow) at basal (subthreshold) Ca2+o (0.5 mM) and effect of repetitive 1-mM increments of Ca2+o (open arrows). (b) Stereoselective mobilization of Ca2+ by 3 mM
l
-Phe but not 3 mM
d
-Phe (closed arrows) at 2.5 mM Ca2+o. (c) Stereoselective mobilization of Ca2+ by 3 mM
l
-Trp but not 3 mM
d
-Trp (closed arrows) at 2.5 mM Ca2+o.
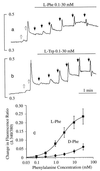
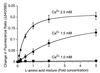
Figure 2
Concentration-dependent mobilization of Ca2+ ions by
l
-Phe and
l
-Trp. In a and b, baseline Ca2+o was 0.5 mM, and 1-mM increments of Ca2+o were applied at the open arrows. The vertical bars in a and b indicate the fluorescence ratios (340/380). (a) Effect of repetitive increments of
l
-Phe (closed arrows, yielding the following final concentrations: 0.1, 0.3, 1.0, 3.0, 10, and 30 mM) in the presence of 2.5 mM Ca2+o. (b) Effect of repetitive increments of
l
-Trp (closed arrows, yielding the following final concentrations: 0.1, 0.3, 1.0, 3.0, 10, and 30 mM) in the presence of 2.5 mM Ca2+o. (c) Concentration-dependent activation of Ca2+ mobilization by
l
-Phe and
d
-Phe at 2.5 mM Ca2+o. The data were derived from cumulative Ca2+i responses (in the form of increased 340/380 fluorescence ratios). The curves were fitted by using the Hill equation. Parameters obtained for
l
-Phe included EC50 (2.2 mM) and the Hill coefficient (1.0).
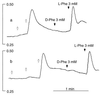
Figure 3
Effect of
l
-Phe on Ca2+ mobilization in the presence of submaximal concentrations of the polycationic CaR agonists spermine and Gd3+o. Ca2+o remained at subthreshold levels (0.5 mM) throughout these experiments. Increments of spermine (0.25 mM) and Gd3+o (5 μM) were applied at the open arrows. The vertical bars indicate the fluorescence ratios (340/380). (a) Stereoselective mobilization of Ca2+ by
l
-Phe but not
d
-Phe (closed arrows) after exposure of HEK-5001 cells to repetitive 0.25-mM increments of spermine. (b) Stereoselective mobilization of Ca2+ by
l
-Phe but not
d
-Phe (closed arrows) after repetitive 5-μM increments of Gd3+o.
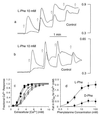
Figure 4
Modulatory effect of
l
-Phe on Ca2+ mobilization induced by Ca2+o and spermine. Fura-2-loaded HEK-293 cells that stably expressed the CaR (HEK-5001 cells) were exposed to physiological saline in the absence or presence of
l
-Phe or
d
-Phe before being loaded into the fluorometer. Baseline Ca2+o was 0.5 mM. The vertical bars in a and b indicate the fluorescence ratios (340/380). (a) Effects of 1-mM increments of Ca2+o (open arrows) on Ca2+ mobilization in the presence or absence of 10 mM
l
-Phe. (b) Effects of 0.25-mM increments of spermine (open arrows) on Ca2+ mobilization in the presence or absence of 10 mM
l
-Phe. (c) Effects of various concentrations of
l
-Phe on the fractional response of the CaR to Ca2+o.
l
-Phe concentrations were as follows: ○, zero; ▵, 1.0 mM; □, 3.0 mM; ●, 10 mM; ▴, 30 mM; ■, 100 mM. (d) Concentration dependence of the stereoselective decrease in the EC50 for Ca2+o induced by
l
-Phe. The following parameters were obtained by curve fitting for
l
-Phe: EC50 = 3.5 mM; Hill coefficient = 1.4. The following parameters were obtained by curve fitting for
d
-Phe: EC50 = 7.4 mM; Hill coefficient = 1.9.
Figure 5
Concentration-dependent effects of
l
-amino acid mixtures on Ca2+ mobilization and sensitivity of the CaR to Ca2+o. HEK-5001 cells were exposed to physiological saline in the absence or presence of various fold (0- to 5-fold) concentrations of a mixture that emulated the fasting plasma levels of the 20 common
l
-amino acids. Concentration-dependent Ca2+ mobilization by the
l
-amino acid mixture was observed at 1.5 mM Ca2+o (●) and 2.5 mM Ca2+o (▴) but not 1.0 mM Ca2+o (■). The data (means ± SEM; n = 4) were derived from cumulative cytoplasmic Ca2+ responses (in the form of increased 340/380 fluorescence ratios).
Comment in
- Allosteric activation of the CaR by L-amino acids.
Kobilka B. Kobilka B. Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4419-20. doi: 10.1073/pnas.97.9.4419. Proc Natl Acad Sci U S A. 2000. PMID: 10781033 Free PMC article. Review. No abstract available.
Similar articles
- L-amino acids regulate parathyroid hormone secretion.
Conigrave AD, Mun HC, Delbridge L, Quinn SJ, Wilkinson M, Brown EM. Conigrave AD, et al. J Biol Chem. 2004 Sep 10;279(37):38151-9. doi: 10.1074/jbc.M406373200. Epub 2004 Jul 2. J Biol Chem. 2004. PMID: 15234970 - Allosteric activation of the extracellular Ca2+-sensing receptor by L-amino acids enhances ERK1/2 phosphorylation.
Lee HJ, Mun HC, Lewis NC, Crouch MF, Culverston EL, Mason RS, Conigrave AD. Lee HJ, et al. Biochem J. 2007 May 15;404(1):141-9. doi: 10.1042/BJ20061826. Biochem J. 2007. PMID: 17212589 Free PMC article. - The Ca2+-sensing receptor: a target for polyamines.
Quinn SJ, Ye CP, Diaz R, Kifor O, Bai M, Vassilev P, Brown E. Quinn SJ, et al. Am J Physiol. 1997 Oct;273(4):C1315-23. doi: 10.1152/ajpcell.1997.273.4.C1315. Am J Physiol. 1997. PMID: 9357776 - L-amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism?
Conigrave AD, Franks AH, Brown EM, Quinn SJ. Conigrave AD, et al. Eur J Clin Nutr. 2002 Nov;56(11):1072-80. doi: 10.1038/sj.ejcn.1601463. Eur J Clin Nutr. 2002. PMID: 12428172 Review. - Physiological significance of L-amino acid sensing by extracellular Ca(2+)-sensing receptors.
Conigrave AD, Mun HC, Brennan SC. Conigrave AD, et al. Biochem Soc Trans. 2007 Nov;35(Pt 5):1195-8. doi: 10.1042/BST0351195. Biochem Soc Trans. 2007. PMID: 17956310 Review.
Cited by
- Physiological changes in the regulation of calcium and phosphorus utilization that occur after the onset of egg production in commercial laying hens.
Garcia-Mejia RA, Sinclair-Black M, Blair LR, Angel R, Jaramillo B, Regmi P, Neupane N, Proszkowiec-Weglarz M, Arbe X, Cavero D, Ellestad LE. Garcia-Mejia RA, et al. Front Physiol. 2024 Sep 25;15:1465817. doi: 10.3389/fphys.2024.1465817. eCollection 2024. Front Physiol. 2024. PMID: 39387099 Free PMC article. - Molecular regulation of calcium-sensing receptor (CaSR)-mediated signaling.
Tian L, Andrews C, Yan Q, Yang JJ. Tian L, et al. Chronic Dis Transl Med. 2024 Apr 29;10(3):167-194. doi: 10.1002/cdt3.123. eCollection 2024 Sep. Chronic Dis Transl Med. 2024. PMID: 39027195 Free PMC article. Review. - Activation of the Calcium-Sensing Receptor by a Subfraction of Amino Acids Contained in Thyroid Drainage Fluid.
Nanoff C, Yang Q, Hellinger R, Hermann M. Nanoff C, et al. ACS Pharmacol Transl Sci. 2024 Jun 28;7(7):1937-1950. doi: 10.1021/acsptsci.3c00350. eCollection 2024 Jul 12. ACS Pharmacol Transl Sci. 2024. PMID: 39022353 - Glutathione-Based Photoaffinity Probe Identifies Caffeine as a Positive Allosteric Modulator of the Calcium-Sensing Receptor.
Matarage Don NNJ, Padmavathi R, Khasro TD, Zaman MRU, Ji HF, Ram JL, Ahn YH. Matarage Don NNJ, et al. ACS Chem Biol. 2024 Jul 19;19(7):1661-1670. doi: 10.1021/acschembio.4c00335. Epub 2024 Jul 8. ACS Chem Biol. 2024. PMID: 38975966 Free PMC article. - Chemotherapy-Induced Changes in Plasma Amino Acids and Lipid Oxidation of Resected Patients with Colorectal Cancer: A Background for Future Studies.
Aquilani R, Brugnatelli S, Maestri R, Iadarola P, Corallo S, Pagani A, Serra F, Bellini A, Buonocore D, Dossena M, Boschi F, Verri M. Aquilani R, et al. Int J Mol Sci. 2024 May 13;25(10):5300. doi: 10.3390/ijms25105300. Int J Mol Sci. 2024. PMID: 38791339 Free PMC article.
References
- Strunz U T, Walsh J H, Grossman M I. Proc Soc Exp Biol Med. 1978;157:440–441. - PubMed
- Isenberg J I, Maxwell V. N Eng J Med. 1978;298:27–29. - PubMed
- Wang C C, Grossman M I. Am J Physiol. 1951;164:527–545. - PubMed
- Meyer J H, Grossman M I. Am J Physiol. 1972;222:1058–1063. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous