How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis - PubMed (original) (raw)
Meta-Analysis
. 2001 Nov 27;165(11):1475-88.
Affiliations
- PMID: 11762571
- PMCID: PMC81663
Meta-Analysis
How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis
H M Schachter et al. CMAJ. 2001.
Abstract
Background: Numerous small clinical trials have been carried out to study the behaviourally defined efficacy and safety of short-acting methylphenidate compared with placebo for attention-deficit disorder (ADD) in individuals aged 18 years and less. However, no meta-analyses that carefully examined these questions have been done. We reviewed the behavioural evidence from all the randomized controlled trials that compared methylphenidate and placebo, and completed a meta-analysis.
Methods: We searched several electronic sources for articles published between 1981 and 1999: MEDLINE, EMBASE, PsychINFO, ERIC, CINAHL, HEALTHSTAR, Biological Abstracts, Current Contents and Dissertation Abstracts. The Cochrane Library Trials Registry and Current Controlled Trials were also consulted. A study was considered eligible for inclusion if it entailed the following: a placebo-controlled randomized trial that involved short-acting methylphenidate and participants aged 18 years or less at the start of the trial who had received any primary diagnosis of ADD that was made in a systematic and reproducible way.
Results: We included 62 randomized trials that involved a total of 2897 participants with a primary diagnosis of ADD (e.g., with or without hyperactivity). The median age of trial participants was 8.7 years, and the median "percent male" composition of trials was 88.1%. Most studies used a crossover design. Using the scores from 2 separate indices, this collection of trials exhibited low quality. Interventions lasted, on average, 3 weeks, with no trial lasting longer than 28 weeks. Each primary outcome (hyperactivity index) demonstrated a significant effect of methylphenidate (effect size reported by teacher 0.78, 95% confidence interval [CI] 0.64-0.91; effect size reported by parent 0.54, 95% CI 0.40-0.67). However, these apparent beneficial effects are tempered by a strong indication of publication bias and the lack of robustness of the findings, especially those involving core ADD features. Methylphenidate also has an adverse event profile that requires consideration. For example, clinicians only need to treat 4 children to identify an episode of decreased appetite.
Interpretation: Short-acting methylphenidate has a statistically significant clinical effect in the short-term treatment of individuals with a diagnosis of ADD aged 18 years and less. However, the extension of this placebo-controlled effect beyond 4 weeks of treatment has not been demonstrated. Exact knowledge of the extent and definition of the short-term behavioural usefulness of methylphenidate is questioned.
Figures
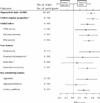
Fig. 1: Effect sizes for the hyperactivity index: teacher ratings (95% confidence interval [CI]). MPH = methylphenidate, ACTRS = Abbreviated Conners Teacher Rating Scale, CTRS = Conners Teacher Rating Scale, SSQ = School Situations Questionnaire. p values for statistical heterogeneity: *p < 0.001, †p = 0.03, ‡p = 0.02, §p = 0.03.
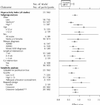
Fig. 2: Effect sizes for the hyperactivity index: parent ratings (95% CI). ACPRS = Abbreviated Conners Parent Rating Scale, CPRS = Conners Parent Rating Scale, HSQ = Home Situations Questionnaire. p values for statistical heterogeneity: *p < 0.001, †p = 0.001, ‡p = 0.008.
Fig. 3: Sensitivity and subgroup analyses of the hyperactivity index: teacher ratings (95% CI). ADHD = attention-deficit hyperactivity disorder (DSM-III-Revised), ADDH = attention-deficit disorder with hyperactivity (DSM-III). p values for statistical heterogeneity: *p = 0.006, †p < 0.001, ‡p = 0.04. §Variance from each trial reduced by 20% to compensate for correlation in crossover phases potentially leading to an underestimate of variance.
Fig. 4: Sensitivity and subgroup analyses of the hyperactivity index: parent ratings (95% CI). *Variance from each trial reduced by 20% to compensate for correlation in crossover phases potentially leading to an underestimate of variance.
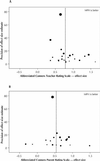
Fig. 5: (A) Funnel plot of methylphenidate effect size for the hyperactivity index (T) versus its precision. The trials' symbols are proportional to sample size (median 32, range 11–161). (B) Funnel plot of methylphenidate effect size for the hyperactivity index (P) versus its precision. The trials' symbols are proportional to sample size (median 37, range 11–161).
Comment in
- Methylphenidate in the treatment of children with attention-deficit hyperactivity disorder.
Vitiello B. Vitiello B. CMAJ. 2001 Nov 27;165(11):1505-6. CMAJ. 2001. PMID: 11762576 Free PMC article. Review. No abstract available. - Review: short acting methylphenidate has short term efficacy in children and adolescents with attention deficit disorder.
Connor DF. Connor DF. Evid Based Ment Health. 2002 May;5(2):50. doi: 10.1136/ebmh.5.2.50. Evid Based Ment Health. 2002. PMID: 12026899 No abstract available. - Treatment of attention-deficit hyperactivity disorder.
Jerome L. Jerome L. CMAJ. 2002 May 14;166(10):1251-2. CMAJ. 2002. PMID: 12041836 Free PMC article. No abstract available.
Similar articles
- Methylphenidate for children and adolescents with autism spectrum disorder.
Sturman N, Deckx L, van Driel ML. Sturman N, et al. Cochrane Database Syst Rev. 2017 Nov 21;11(11):CD011144. doi: 10.1002/14651858.CD011144.pub2. Cochrane Database Syst Rev. 2017. PMID: 29159857 Free PMC article. Review. - Immediate-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults.
Epstein T, Patsopoulos NA, Weiser M. Epstein T, et al. Cochrane Database Syst Rev. 2014 Sep 18;(9):CD005041. doi: 10.1002/14651858.CD005041.pub2. Cochrane Database Syst Rev. 2014. PMID: 25230710 Updated. Review. - Treatment of attention-deficit/hyperactivity disorder.
Jadad AR, Boyle M, Cunningham C, Kim M, Schachar R. Jadad AR, et al. Evid Rep Technol Assess (Summ). 1999 Nov;(11):i-viii, 1-341. Evid Rep Technol Assess (Summ). 1999. PMID: 10790990 Free PMC article. - Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis.
Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, Atkinson LZ, Tessari L, Banaschewski T, Coghill D, Hollis C, Simonoff E, Zuddas A, Barbui C, Purgato M, Steinhausen HC, Shokraneh F, Xia J, Cipriani A. Cortese S, et al. Lancet Psychiatry. 2018 Sep;5(9):727-738. doi: 10.1016/S2215-0366(18)30269-4. Epub 2018 Aug 7. Lancet Psychiatry. 2018. PMID: 30097390 Free PMC article. - The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials.
Catalá-López F, Hutton B, Núñez-Beltrán A, Page MJ, Ridao M, Macías Saint-Gerons D, Catalá MA, Tabarés-Seisdedos R, Moher D. Catalá-López F, et al. PLoS One. 2017 Jul 12;12(7):e0180355. doi: 10.1371/journal.pone.0180355. eCollection 2017. PLoS One. 2017. PMID: 28700715 Free PMC article.
Cited by
- Response to methylphenidate by adult and pediatric patients with attention-deficit/hyperactivity disorder: the Spanish multicenter DIHANA study.
Valdizán-Usón JR, Cánovas-Martínez A, De Lucas-Taracena MT, Díaz-Atienza F, Eddy-Ives LS, Fernández-Jaén A, Fernández-Pérez M, García-Giral M, García-Magán P, Garraus-Oneca M, Idiazábal-Alecha MA, López-Benito M, Lorenzo-Sanz G, Martínez-Antón J, Martínez-Granero MA, Montañés-Rada F, Mulas-Delgado F, Ochando-Perales G, Ortega-García E, Pelaz-Antolín A, Ramos-Quiroga JA, Ruiz-Sanz FC, Vaquerizo-Madrid J, Yusta-Izquierdo A. Valdizán-Usón JR, et al. Neuropsychiatr Dis Treat. 2013;9:211-8. doi: 10.2147/NDT.S35836. Epub 2013 Feb 8. Neuropsychiatr Dis Treat. 2013. PMID: 23430373 Free PMC article. - The Effectiveness of Mindfulness Training for Children with ADHD and Mindful Parenting for their Parents.
van der Oord S, Bögels SM, Peijnenburg D. van der Oord S, et al. J Child Fam Stud. 2012 Feb;21(1):139-147. doi: 10.1007/s10826-011-9457-0. Epub 2011 Feb 2. J Child Fam Stud. 2012. PMID: 22347788 Free PMC article. - Drug and non-drug treatments of children with ADHD and tic disorders.
Poncin Y, Sukhodolsky DG, McGuire J, Scahill L. Poncin Y, et al. Eur Child Adolesc Psychiatry. 2007 Jun;16 Suppl 1:78-88. doi: 10.1007/s00787-007-1010-8. Eur Child Adolesc Psychiatry. 2007. PMID: 17665286 Review. - Effects of multisensory stop signals on alcohol-induced disinhibition in adults with ADHD.
D'Agostino AR, Wesley MJ, Brown J, Fillmore MT. D'Agostino AR, et al. Exp Clin Psychopharmacol. 2019 Jun;27(3):247-256. doi: 10.1037/pha0000251. Epub 2019 Jan 10. Exp Clin Psychopharmacol. 2019. PMID: 30628812 Free PMC article. - Is S100B Involved in Attention-Deficit/Hyperactivity Disorder (ADHD)? Comparisons with Controls and Changes Following a Triple Therapy Containing Methylphenidate, Melatonin and ω-3 PUFAs.
Ouadih-Moran M, Muñoz-Hoyos A, D'Marco L, Molina-Carballo A, Seiquer I, Checa-Ros A. Ouadih-Moran M, et al. Nutrients. 2023 Jan 31;15(3):712. doi: 10.3390/nu15030712. Nutrients. 2023. PMID: 36771418 Free PMC article. Clinical Trial.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). Washington: The Association; 1994.
- Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of Attention-Deficit/Hyperactivity Disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA 1998; 279: 1100-7. - PubMed
- Jadad AR, Boyle M, Cunningham C, Kim M, Schachar R. Treatment of Attention-Deficit/Hyperactivity Disorder. Prepared for Agency of Healthcare Research and Quality. Rockville (MD): The Agency; 1999.
- Elia J, Ambrosini P, Rapoport J. Treatment of Attention-Deficit/Hyperactivity Disorder. N Engl J Med 1999;340:780-8. - PubMed
- Barkley RA, editor. Attention-deficit hyperactivity disorder. A handbook for diagnosis and treatment. 2nd ed. New York: The Guilford Press; 1998.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical