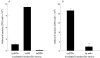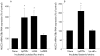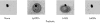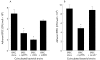Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro - PubMed (original) (raw)
Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro
D R Mack et al. Gut. 2003 Jun.
Abstract
Background: Mucins are large complex glycoproteins that protect intestinal mucosal surfaces by limiting access of environmental matter to their epithelial cells. Several mucin genes have been described, including MUC3 that is a membrane associated mucin of the small intestine. Increased MUC3 mRNA transcription is induced by incubation of intestinal epithelial cells with a Lactobacillus strain known to be adherent to them.
Aims: To determine whether increased epithelial cell MUC3 mucin expression in response to Lactobacillus strains results in increased extracellular secretion of MUC3 mucins and the importance of epithelial cell adherence in modulation of MUC3 mucin expression.
Methods: HT29 cells grown to enhance expression of MUC3 mucins were incubated with selected Lactobacillus strains. Spent cell culture medium was collected for detection of secreted MUC3 mucins using dot blot immunoassay with a generated MUC3 antibody. Post-incubation HT29 cell RNA was collected for analysis of MUC3 expression by northern blot analysis using a MUC3 cDNA probe. In vitro binding studies using Lactobacillus strains incubated alone or coincubated with enteropathogenic Escherichia coli strain E2348/69 were used for adherence and inhibition of adherence studies, respectively.
Results: Lactobacillus strains with minimal ability to adhere to HT29 cells failed to induce upregulation of mucin gene expression. There was a direct correlation between upregulation of MUC3 mucin mRNA expression and extracellular secretion of MUC3 mucin. The same Lactobacillus strains that increased extracellular secretion of MUC3 mucin led to reduced adherence of enteropathogen E coli E2348/69 during coincubation experiments.
Conclusion: Probiotic microbes induce MUC3 mucin transcription and translation with extracellular secretion of the MUC3 mucins. Epithelial cell adherence enhances the effects of probiotics on eukaryotic mucin expression.
Figures
Figure 1
(A) Lactobacillus adherence to HT29 cells. Lactobacillus strains 106 colony forming unit (CFU)/well were incubated for four hours with HT29 cells grown in glucose free galactose containing cell culture medium to enhance MUC3 expression. Non-adherent bacteria were washed off (×4) and adherent bacteria were quantified by CFU determination on MRS agar plates. Lp299v, Lactobacillus plantarum strain 299v; LrGG, L rhamnosus strain GG; LaDDS, L acidophilus strain DDS-1. (B) Similar experiments were performed using the parent Lp299v strain and its adhesin negative genetic mutant Lp adh− strain. In both, five experiments were run in triplicate and results are expressed as mean (SEM).
Figure 2
Mucin mRNA expression induced by Lactobacillus strains. Lactobacilli (4.5×1010 colony forming units) were added to flasks in an equivalent amount as cell surface contact area of 12 well plates. After one hour of incubation, northern blots were hybridised using random-primed P labelled cDNA probe to the MUC3 tandem repeat. Mucin mRNA levels were quantified by area integration of Phosphor screen autoradiography. Results were normalised to 28s RNA levels on agarose gels used for northern blots. Results are expressed as means (SEM) of five experiments for HT29 cells were grown in a glucose free galactose containing cell culture medium to enhance MUC3 expression. As shown in (A), MUC3 expression for cells incubated with Lactobacillus plantarum strain 299v (Lp299v) and L rhamnosus strain GG (LrGG) were increased compared with controls without bacteria added and HT29 cells with L acidophilus strain DDS-1 (LaDDS) added (*p<0.05). (B) MUC3 mRNA expression was increased for Lp299v compared with the ligand negative Lp adh− strain (*p<0.05).
Figure 3
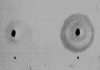
Immunoassay of HT29 spent cell culture medium. Immune serum generated against a 9 mer peptide (TSRRTTRIT) of deduced MUC3 sequence was incubated with MUC2 (A) and MUC3 mucins (B) isolated from HT29 cell culture medium. Mucin protein (20 μg) was blotted onto nitrocellulose. A 1:100 dilution of mouse antisera was added. After washings, bound antibody was detected by a horseradish peroxidase colorisation reaction. A black dot in each frame is pencil marking used to indicate where medium was blotted initially. High reactivity was observed for spent culture medium of cells grown in galactose containing medium to enhance MUC3 expression. In contrast, there was less reactivity to material from spent culture medium of HT29 cells grown in a glucose containing medium that is associated with high MUC2 mucin mRNA expression and low MUC3 mucin mRNA expression.
Figure 4
Indirect immunofluorescence of human duodenum. The photomicrograph using mouse antihuman MUC3 mucin antibody as the primary antibody is shown. The cross section view of a villus from a biopsy taken from the second part of the duodenum shows fluorescence of the epithelial cell cytoplasm with increased fluorescence at the surface of the epithelial cells (arrows). Controls using preimmune serum, FITC conjugate alone, and phosphate buffered saline were negative (not shown). Magnification 40×.
Figure 5
Immunoassay for MUC3 mucins in spent cell culture medium from Lactobacillus strains. HT29 cells were grown in glucose free galactose containing medium to enhance MUC3 expression and incubated with equivalent colony forming units of different Lactobacillus strains. Cell culture medium (10 μg protein) was blotted onto nitrocellulose. A 1:100 dilution of mouse anti-MUC3 antisera was added. After washings, bound antibody was detected using a peroxidase colorisation reaction. A black dot in each frame is pencil marking used to indicate where medium was blotted initially. Greater immunoreactive MUC3 mucin was detected in cell culture medium incubated with Lactobacillus plantarum strain 299v (Lp299v) and L rhamnosus strain GG (LrGG) than with L acidophilus strain DDS-1 (LaDDS) and control cells without a probiotic added (none).
Figure 6
Comparative inhibition of enteropathogenic Escherichia coli (EPEC) adherence to MUC3 expressing HT29 cells. Each well received 109 colony forming units (CFU)/well Lactobacillus that were incubated at 37°C with the HT29 cells for an hour prior to the addition of 106 CFU/well of EPEC E2348/69. After a three hour incubation, EPEC adherent to cells were quantified by determining CFU on MacConkey agar. Results are expressed as means (SEM) of at least five independent experiments performed in triplicate. As shown in (A), both Lactobacillus plantarum strain 299v (Lp299v) and L rhamnosus strain GG (LrGG) decreased adherence of EPEC compared with controls of EPEC alone but L acidophilus strain DDS-1 (LaDDS) did not (*p<0.05). (B) The ligand negative strain Lp adh− showed less adherence than the parent ligand positive strain Lp299v (*p<0.05).
Similar articles
- Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression.
Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Mack DR, et al. Am J Physiol. 1999 Apr;276(4):G941-50. doi: 10.1152/ajpgi.1999.276.4.G941. Am J Physiol. 1999. PMID: 10198338 - The conserved TFLK motif of mammary-associated serum amyloid A3 is responsible for up-regulation of intestinal MUC3 mucin expression in vitro.
Mack DR, McDonald TL, Larson MA, Wei S, Weber A. Mack DR, et al. Pediatr Res. 2003 Jan;53(1):137-42. doi: 10.1203/00006450-200301000-00023. Pediatr Res. 2003. PMID: 12508093 - Human serum amyloid A3 peptide enhances intestinal MUC3 expression and inhibits EPEC adherence.
Larson MA, Wei SH, Weber A, Mack DR, McDonald TL. Larson MA, et al. Biochem Biophys Res Commun. 2003 Jan 10;300(2):531-40. doi: 10.1016/s0006-291x(02)02901-7. Biochem Biophys Res Commun. 2003. PMID: 12504116 - Biosynthesis of intestinal mucins: MUC1, MUC2, MUC3 and more.
Van Klinken BJ, Tytgat KM, Büller HA, Einerhand AW, Dekker J. Van Klinken BJ, et al. Biochem Soc Trans. 1995 Nov;23(4):814-8. doi: 10.1042/bst0230814. Biochem Soc Trans. 1995. PMID: 8654844 Review. No abstract available. - Lactobacillus adhesion to mucus.
Van Tassell ML, Miller MJ. Van Tassell ML, et al. Nutrients. 2011 May;3(5):613-36. doi: 10.3390/nu3050613. Epub 2011 May 20. Nutrients. 2011. PMID: 22254114 Free PMC article. Review.
Cited by
- The role of probiotics in management of irritable bowel syndrome.
Borowiec AM, Fedorak RN. Borowiec AM, et al. Curr Gastroenterol Rep. 2007 Oct;9(5):393-400. doi: 10.1007/s11894-007-0048-6. Curr Gastroenterol Rep. 2007. PMID: 17991340 Review. - The Importance of Accurate Early Diagnosis and Eradication in Helicobacter pylori Infection: Pictorial Summary Review in Children and Adults.
Marginean CM, Cioboata R, Olteanu M, Vasile CM, Popescu M, Popescu AIS, Bondari S, Pirscoveanu D, Marginean IC, Iacob GA, Popescu MD, Stanciu M, Mitrut P. Marginean CM, et al. Antibiotics (Basel). 2022 Dec 29;12(1):60. doi: 10.3390/antibiotics12010060. Antibiotics (Basel). 2022. PMID: 36671261 Free PMC article. Review. - Beneficial insights into postbiotics against colorectal cancer.
Song D, Wang X, Ma Y, Liu NN, Wang H. Song D, et al. Front Nutr. 2023 Mar 10;10:1111872. doi: 10.3389/fnut.2023.1111872. eCollection 2023. Front Nutr. 2023. PMID: 36969804 Free PMC article. Review. - Probiotics and prebiotics in inflammatory bowel disease: microflora 'on the scope'.
Damaskos D, Kolios G. Damaskos D, et al. Br J Clin Pharmacol. 2008 Apr;65(4):453-67. doi: 10.1111/j.1365-2125.2008.03096.x. Epub 2008 Feb 12. Br J Clin Pharmacol. 2008. PMID: 18279467 Free PMC article. Review. - Are probiotics useful in Helicobacter pylori eradication?
Homan M, Orel R. Homan M, et al. World J Gastroenterol. 2015 Oct 7;21(37):10644-53. doi: 10.3748/wjg.v21.i37.10644. World J Gastroenterol. 2015. PMID: 26457024 Free PMC article. Review.
References
- Hecht G. Innate mechanisms of epithelial host defense: spotlight on intestine. Am J Physiol 1999;277:C351–8. - PubMed
- Dai D, Nanthkumar N, Newburg DS, et al. Role of oligosaccharides and glycoconjugates in intestinal host defense. J Pediatr Gastroenterol Nutr 2000;30:S23–33. - PubMed
- Yolken RH, Ojeh C, Khatri IA, et al. Intestinal mucins inhibit rotavirus replication in an oligosaccharide-dependent manner. J Infect Dis 1994;169:1002–6. - PubMed
- Ho SB, Niehans GA, Lyftogt C, et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res 1993;53:641–51. - PubMed
- Chang SK, Dohrman AF, Basbaum CB, et al. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology 1994;107:28–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials