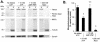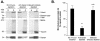Cytoplasmic dynein mediates adenovirus binding to microtubules - PubMed (original) (raw)
Cytoplasmic dynein mediates adenovirus binding to microtubules
Samir A Kelkar et al. J Virol. 2004 Sep.
Abstract
During infection, adenovirus (Ad) capsids undergo microtubule-dependent retrograde transport as part of a program of vectorial transport of the viral genome to the nucleus. The microtubule-associated molecular motor, cytoplasmic dynein, has been implicated in the retrograde movement of Ad. We hypothesized that cytoplasmic dynein constituted the primary mode of association of Ad with microtubules. To evaluate this hypothesis, an Ad-microtubule binding assay was established in which microtubules were polymerized with taxol, combined with Ad in the presence or absence of microtubule-associated proteins (MAPs), and centrifuged through a glycerol cushion. The addition of purified bovine brain MAPs increased the fraction of Ad in the microtubule pellet from 17.3% +/- 3.5% to 80.7% +/- 3.8% (P < 0.01). In the absence of tubulin polymerization or in the presence of high salt, no Ad was found in the pellet. Ad binding to microtubules was not enhanced by bovine brain MAPs enriched for tau protein or by the addition of bovine serum albumin. Enhanced Ad-microtubule binding was also observed by using a fraction of MAPs purified from lung A549 epithelial cell lysate which contained cytoplasmic dynein. Ad-microtubule interaction was sensitive to the addition of ATP, a hallmark of cytoplasmic dynein-dependent microtubule interactions. Immunodepletion of cytoplasmic dynein from the A549 cell lysate abolished the MAP-enhanced Ad-microtubule binding. The interaction of Ad with both dynein and dynactin complexes was demonstrated by coimmunoprecipitation. Partially uncoated capsids isolated from cells 40 min after infection also exhibited microtubule binding. In summary, the primary mode of Ad attachment to microtubules occurs though cytoplasmic dynein-mediated binding.
Copyright 2004 American Society for Microbiology
Figures
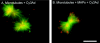
FIG. 1.
Visualization of MAP-mediated Ad interaction with microtubules. Purified fluorescein-conjugated bovine brain tubulin was polymerized into microtubules by the addition of taxol. Cy3-conjugated Ad serotype 5 (Cy3Ad) capsids were incubated with microtubules in the absence or presence of purified bovine brain MAPs, pelleted, resuspended, and placed on a coverslip. Microtubules (green) and Cy3-conjugated Ad capsids (red) were evaluated by fluorescence microscopy. (A) Ad interaction with microtubules without MAPs. (B) Ad interaction with microtubules in the presence of MAPs. Bar = 5 μm.
FIG. 2.
Quantitative analysis of MAP-dependent Ad interaction with microtubules. Purified bovine brain tubulin dimers were polymerized into microtubules as described in the legend of Fig. 1. Taxol-polymerized microtubules or unpolymerized tubulin was incubated in the absence or presence of purified bovine brain MAPs or tau in the presence or absence of 500 mM NaCl. Cy3-conjugated Ad serotype 5 capsids were incubated under each reaction condition, placed over a glycerol cushion, and centrifuged. Supernatant (S) and pellet (P) were then analyzed by gel electrophoresis. (A) Fluorescence scan and Coomassie blue stain. Cy3-conjugated Ad capsid protein distributions in the supernatant and pellet were evaluated by fluorescence scan: hexon (106 kDa), penton base (74 kDa), and fiber (62 kDa) are shown. Tubulin and MAP distributions in the supernatant and pellet were evaluated by Coomassie blue stain: high-molecular-weight MAPs (>200 kDa), tau (65 kDa), and tubulin (55 kDa) are shown. (B) Quantitation of hexon fluorescence signal in pellet by digital analysis as a percentage of total (supernatant plus pellet) signal. Data are presented as the means and standard deviations of four separate experiments. *, significant difference compared to control (P < 0.01); **, no significant difference compared to control (_P_ > 0.1).
FIG. 3.
Nucleotide-sensitive Ad interaction with microtubules. Purified fluorescein-conjugated bovine brain tubulin was polymerized into microtubules by the addition of taxol. Fluorescein-microtubule solution was then placed over a cushion and centrifuged, and the pellet was resuspended with taxol. A549 lung epithelial cells were harvested and lysed, and polymerized fluorescein microtubules were added to the lysate with no exogenous ATP, with ATP, or with AMP-PNP. Fluorescein-microtubule solution was then placed over a cushion and centrifuged, and the pellet was resuspended with taxol. The pellets from the reactions were resuspended, and Cy3-conjugated Ad serotype 5 capsids were incubated with the MAP-bound microtubules and assessed by fluorescence microscopy. Microtubules (green) and Cy3-conjugated Ad capsids (red) were evaluated by fluorescence microscopy. (A) Ad interaction with microtubules without exogenous nucleotide. (B) Ad interaction with microtubules in the presence of ATP. (C) Ad interaction with microtubules in the presence of AMP-PNP. Bar = 5 μm.
FIG. 4.
Quantitative assessment of nucleotide-dependent binding of Ad with microtubules and MAPs purified from A549 cell lysate. A549 lung epithelial cell lysate was prepared as described in the legend of Fig. 3. Microtubules and MAPs from cell lysate were purified after incubation with taxol as described in Materials and Methods. Pellets containing polymerized microtubules and MAPs were resuspended in PEM-G containing no exogenous ATP, 10 mM ATP, or 10 mM AMP-PNP (a nonhydrolyzable analog of ATP). Cy3-conjugated Ad serotype 5 capsids were added to each reaction mixture, placed over a cushion buffer composed of PEM with 60% (vol/vol) glycerol, and centrifuged as described in Materials and Methods. Supernatant (S) and pellet (P) were then analyzed by gel electrophoresis and Western analysis. (A) Fluorescence scan and Coomassie blue stain. Cy3-conjugated Ad capsid protein distributions in the supernatant and pellet were evaluated by fluorescence scanning: hexon (106 kDa), penton base (74 kDa), and fiber (62 kDa) are shown. Tubulin and MAP distributions in the supernatant and pellet were evaluated by Coomassie blue staining: high-molecular-weight MAPs (>200 kDa) and tubulin (55 kDa) are shown. The presence of dynein (74 kDa) in the supernatant and pellet was evaluated by Western analysis. (B) Quantitation of hexon fluorescence signal in pellet by digital analysis as a percentage of total (supernatant plus pellet) signal. Data are presented as the means and standard deviations of four separate experiments. *, significant statistical difference compared to control (P < 0.05); **, no significant statistical difference compared to control (_P_ > 0.7).
FIG. 5.
Contribution of cytoplasmic dynein in MAP-enhanced Ad interaction with human cell line-derived microtubules. A549 lung epithelial cells (10 confluent 150-mm dishes) were harvested and lysed by sonication in PEM buffer on ice. Antibodies for immunodepletion of the lysate were added to cell lysate and incubated (4 to 8 h at 4°C). Immunodepletion antibodies included ascites fluid containing monoclonal anticytoplasmic dynein antibody 74.1 and anti-κ light chain. Protein A/G beads were then added to clear antibody and target protein. Endogenous tubulin was polymerized into microtubules by the addition of taxol (40 μM). Taxol-treated cell lysate was then placed over a cushion composed of PEM buffer with 60% (vol/vol) glycerol plus taxol (40 μM) and centrifuged (100,000 × g for 40 min). Pellets containing polymerized microtubules and MAPs were resuspended in PEM-G. Cy3-conjugated Ad serotype 5 capsids (109 particles) were incubated with microtubules and MAPs, placed over a cushion composed of PEM buffer with 60% (vol/vol) glycerol plus taxol (40 μM), and centrifuged (100,000 × g for 40 min). Supernatant (S) and pellet (P) were then analyzed by gel electrophoresis. (A) Fluorescence scan and Coomassie blue stain. Cy3-conjugated Ad capsid protein distribution in the supernatant and pellet was evaluated by a fluorescence scan: hexon (106 kDa), penton base (74 kDa), and fiber (62 kDa) are shown. Tubulin and MAP distributions in the supernatant and pellet were evaluated by Coomassie blue staining: high-molecular-weight MAPs (>200 kDa) and tubulin (55 kDa) are shown. The presence of dynein (74 kDa) in the supernatant and pellet was evaluated by Western analysis. (B) Quantitation of hexon fluorescence signal in the pellet by digital analysis as a percentage of total (supernatant plus pellet) signal. Data are presented as the means and standard deviations of four separate experiments. *, significant statistical difference compared to control (P < 0.01); **, no significant statistical difference compared to control (_P_ > 0.9).
FIG. 6.
Coimmunoprecipitation of cytoplasmic dynein, dynamitin, and Ad. A549 cell lysate was prepared as described in Materials and Methods. Cell lysate in PEM was incubated with either protein A/G or protein L agarose beads for 1 h at 4°C and centrifuged to preclear nonspecific agarose bead binding proteins (three times). Cy3-conjugated Ad serotype 5 capsids (2 × 1010 particles) were added to cell lysate and incubated (40 min at 22°C). Immunoprecipitation antibodies (mouse ascites fluid containing monoclonal anticytoplasmic dynein antibody 74.1, mouse ascites fluid containing anti-horseradish peroxidase antibody, human sera previously characterized to neutralize in vitro Ad infection, and human serum previously demonstrated to be nonneutralizing for in vitro infection by Ad) were incubated for 4 to 8 h at 4°C. Samples containing mouse antibodies were incubated with protein A/G (1 h at 4°C) and centrifuged to isolate antibody and target protein. Samples containing human sera were incubated with protein L beads (1 h, 4°C) and centrifuged to isolate antibody and target protein. Beads were then washed three times with PEM (with 10% glycerol). Immunoprecipitated proteins were analyzed by gel electrophoresis (A) Fluorescence scan and immunoblot of antidynein immunoprecipitated proteins. Cy3-conjugated Ad capsid protein presence was evaluated by a fluorescence scan: hexon (106 kDa), penton base (74 kDa), and fiber (62 kDa) are shown. The presence of dynein (74 kDa) and dynamitin (50 kDa) was evaluated by Western analysis. (B) Immunoblot and fluorescence scan of anti-Ad immunoprecipitated proteins. The presence of dynein (74 kDa) and dynamitin (50 kDa) was evaluated by Western analysis. Cy3-conjugated Ad capsid protein presence was evaluated by a fluorescence scan: hexon (106 kDa) penton base (74 kDa), and fiber (62 kDa) are shown.
FIG. 7.
Intracellular Ad particles interact with human cell line-derived microtubules in a nucleotide-sensitive manner. A549 cells were cultured to 80% confluence as described in Materials and Methods. Cells were washed three times with serum-free DMEM and infected with Ad5 (1,000 particles/cell) for 10 min at 37°C in serum-free DMEM. Cells were washed three times with serum containing DMEM and incubated for 40 min at 37°C. Ad-infected A549 cell lysate was then harvested as previously described. To test the interaction of intracellular Ad capsids with A549-derived microtubules, endogenous tubulin of Ad-infected A549 cell lysate was polymerized into microtubules by the addition of taxol (40 μM) and incubated for 80 min at 22°C. Reactions were centrifuged as described previously. The presence of Ad capsid in the supernatant (S) and pellet (P) was evaluated by SDS-PAGE and Western analysis.
Similar articles
- A common mechanism for cytoplasmic dynein-dependent microtubule binding shared among adeno-associated virus and adenovirus serotypes.
Kelkar S, De BP, Gao G, Wilson JM, Crystal RG, Leopold PL. Kelkar S, et al. J Virol. 2006 Aug;80(15):7781-5. doi: 10.1128/JVI.00481-06. J Virol. 2006. PMID: 16840360 Free PMC article. - Nucleotide-dependent behavior of single molecules of cytoplasmic dynein on microtubules in vitro.
Miura M, Matsubara A, Kobayashi T, Edamatsu M, Toyoshima YY. Miura M, et al. FEBS Lett. 2010 Jun 3;584(11):2351-5. doi: 10.1016/j.febslet.2010.04.016. Epub 2010 Apr 13. FEBS Lett. 2010. PMID: 20394748 - Cytoplasmic dynein ATPase activity is regulated by dynactin-dependent phosphorylation.
Kumar S, Lee IH, Plamann M. Kumar S, et al. J Biol Chem. 2000 Oct 13;275(41):31798-804. doi: 10.1074/jbc.M000449200. J Biol Chem. 2000. PMID: 10921911 - [Dynein and dynactin as organizers of the system of cell microtubules].
Burakov AV, Nadezhdina ES. Burakov AV, et al. Ontogenez. 2006 Sep-Oct;37(5):323-39. Ontogenez. 2006. PMID: 17066975 Review. Russian. - Dynein motility: four heads are better than two.
McGrath JL. McGrath JL. Curr Biol. 2005 Dec 6;15(23):R970-2. doi: 10.1016/j.cub.2005.11.021. Curr Biol. 2005. PMID: 16332531 Review.
Cited by
- Adenovirus-Derived Nano-Capsid Platforms for Targeted Delivery and Penetration of Macromolecules into Resistant and Metastatic Tumors.
Benhaghnazar RL, Medina-Kauwe L. Benhaghnazar RL, et al. Cancers (Basel). 2023 Jun 19;15(12):3240. doi: 10.3390/cancers15123240. Cancers (Basel). 2023. PMID: 37370850 Free PMC article. Review. - Could artificial intelligence revolutionize the development of nanovectors for gene therapy and mRNA vaccines?
Hasanzadeh A, Hamblin MR, Kiani J, Noori H, Hardie JM, Karimi M, Shafiee H. Hasanzadeh A, et al. Nano Today. 2022 Dec;47:101665. doi: 10.1016/j.nantod.2022.101665. Epub 2022 Nov 7. Nano Today. 2022. PMID: 37034382 Free PMC article. - Adenovirus core protein V reinforces the capsid and enhances genome release from disrupted particles.
Martín-González N, Gómez-González A, Hernando-Pérez M, Bauer M, Greber UF, San Martín C, de Pablo PJ. Martín-González N, et al. Sci Adv. 2023 Apr 7;9(14):eade9910. doi: 10.1126/sciadv.ade9910. Epub 2023 Apr 7. Sci Adv. 2023. PMID: 37027464 Free PMC article. - Nuclear entry and egress of parvoviruses.
Mattola S, Aho V, Bustamante-Jaramillo LF, Pizzioli E, Kann M, Vihinen-Ranta M. Mattola S, et al. Mol Microbiol. 2022 Oct;118(4):295-308. doi: 10.1111/mmi.14974. Epub 2022 Aug 24. Mol Microbiol. 2022. PMID: 35974704 Free PMC article. Review. - Nano-Particles Carried by Multiple Dynein Motors Self-Regulate Their Number of Actively Participating Motors.
Halbi G, Fayer I, Aranovich D, Gat S, Bar S, Erukhimovitch V, Granek R, Bernheim-Groswasser A. Halbi G, et al. Int J Mol Sci. 2021 Aug 18;22(16):8893. doi: 10.3390/ijms22168893. Int J Mol Sci. 2021. PMID: 34445598 Free PMC article.
References
- Alonso, C., J. Miskin, B. Hernaez, P. Fernandez-Zapatero, L. Soto, C. Canto, I. Rodriguez-Crespo, L. Dixon, and J. M. Escribano. 2001. African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J. Virol. 75:9819-9827. - PMC - PubMed
- Acsadi, G., R. A. Anguelov, H. Yang, G. Toth, R. Thomas, A. Jani, Y. Wang, E. Ianakova, S. Mohammad, R. A. Lewis, and M. E Shy. 2002. Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum. Gene Ther. 13:1047-1059. - PubMed
- Baumgartner, B. J., and H. D Shine. 1998. Neuroprotection of spinal motoneurons following targeted transduction with an adenoviral vector carrying the gene for glial cell line-derived neurotrophic factor. Exp Neurol. 153:102-112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous