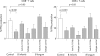Modulation of human dendritic cell phenotype and function by probiotic bacteria - PubMed (original) (raw)
Modulation of human dendritic cell phenotype and function by probiotic bacteria
A L Hart et al. Gut. 2004 Nov.
Abstract
Background: "Probiotic" bacteria are effective in treating some inflammatory bowel diseases. However which bacteria confer benefit and mechanisms of action remain poorly defined. Dendritic cells, which are pivotal in early bacterial recognition, tolerance induction, and shaping of T cell responses, may be central in mediating the effects of these bacteria.
Aims: To assess effects of different probiotic bacteria on dendritic cell function.
Methods: Human intestinal lamina propria mononuclear cells, whole blood, or an enriched blood dendritic cell population were cultured with cell wall components of the eight bacterial strains in the probiotic preparation VSL#3 (four lactobacilli, three bifidobacteria, and one streptococcal strains). Dendritic cells were identified and changes in dendritic cell maturation/costimulatory markers and cytokine production in response to probiotic bacteria were analysed by multicolour flow cytometry, in addition to subsequent effects on T cell polarisation.
Results: VSL#3 was a potent inducer of IL-10 by dendritic cells from blood and intestinal tissue, and inhibited generation of Th1 cells. Individual strains within VSL#3 displayed distinct immunomodulatory effects on dendritic cells; the most marked anti-inflammatory effects were produced by bifidobacteria strains which upregulated IL-10 production by dendritic cells, decreased expression of the costimulatory molecule CD80, and decreased interferon-gamma production by T cells. VSL#3 diminished proinflammatory effects of LPS by decreasing LPS induced production of IL-12 while maintaining IL-10 production.
Conclusions: Probiotic bacteria differ in their immunomodulatory activity and influence polarisation of immune responses at the earliest stage of antigen presentation by dendritic cells.
Figures
Figure 1
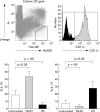
Cytokine production by blood dendritic cells. Dendritic cells (DC) were identified by multicolour flow cytometry as an HLA−DR+ lineage negative (CD3−, CD14−, CD16−, CD19−, CD34−, and CD56−) population. Within this DC gate, CD11c+ and CD11c− DC subsets were identified. The cytokine production by these DC subsets was assessed by intracellular cytokine staining and super-enhanced Dmax normalised subtraction. One parameter histograms for the intracellular staining of IL-10 with and without added monensin are shown. In this example, cultures were stimulated with VSL#3 probiotic bacteria. Intracellular cytokine production was determined by subtracting labelling of the sample without monensin from labelling of the sample with monensin using WinList software. In the histograms with subtraction percentages, the outline of the histogram indicates staining of monensin treated cells and the shaded area represents the proportion of cytokine positive cells after the subtraction. Cytokine detection was competitively inhibited by unlabelled antibody of the same clone as that used for cytokine detection, but not by unlabelled antibody of irrelevant specificity.
Figure 2
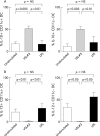
Enhanced IL-10 production and decreased IL-12 production by blood DC in the presence of probiotic bacteria (VSL#3). Whole blood was incubated with control medium alone, VSL#3 probiotic bacteria (at a dose equivalent to 108 CFU/ml) or LPS (1 μg/ml) for four hours and (A) IL-10 production and (B) IL-12 production was assessed by intracellular cytokine staining. The proportion of cytokine staining CD11c+ and CD11c− DC is shown (mean (SEM), n = 9−13).
Figure 3
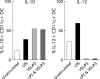
Co-culture of VSL#3 with LPS decreased the proinflammatory effects of LPS. In whole blood, VSL#3 at 108 CFU/ml and LPS at 1 μg/ml were co-cultured and cytokine production assessed by intracellular staining. The proportion of CD11c+ DC producing IL-10 and IL-12 in these co-cultures is shown. The data shown are representative of three independent experiments.
Figure 4
Enhanced IL-10 production by an enriched DC population in the presence of probiotic bacteria (VSL#3). (A) An enriched DC population (metrizamide separated) was cultured with control medium alone, VSL#3 probiotic bacteria (at a dose equivalent to 108 CFU/ml) or LPS (1 μg/ml) for four hours and the IL-10 production assessed by intracellular cytokine staining. (B) The concentration of IL-10 in the supernatants of enriched DC cultured with control medium, VSL#3 (at a dose equivalent to 108 CFU/ml) or LPS (1 μg/ml) was measured by ELISA at four hours and 16 hours. The results are representative of three independent experiments.
Figure 5
Cytokine production by lamina propria CD11c+ DC extracted from mucosal tissue. DC were identified by multicolour flow cytometry as HLA−DR+ lineage-negative (CD3−, CD14−, CD16−, CD19−, CD34−, and CD56−). Within this gate, CD11c+ DC were identified. The effect of control medium alone, VSL#3 (at a dose equivalent to 108 CFU/ml) or LPS (1 μg/ml) on IL-10 and IL-12 production by intestinal derived CD11c+ DC is shown (mean (SEM), n = 9−11).
Figure 6
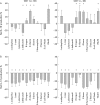
Effect of individual bacterial strains on IL-12 and IL-10 production by CD11c+ and CD11c− DC. When addition of bacteria to the culture increased cytokine production compared with that seen in the absence of any added stimulus for a given individual, the proportion of cytokine producing cells was taken as a net positive value. If the bacteria decreased cytokine production compared with that seen in the absence of bacteria, the proportion and absolute number of cells was taken as a net negative value. The data indicate the mean (SEM) for three to six independent experiments. (A) The effect of the different probiotic strains at 108 CFU/ml on IL-10 production by CD11c+ and CD11c− DC. (B) The effect of the different probiotic strains at 108 CFU/ml on IL-12 production by CD11c+ and CD11c− DC. * Significant changes in cytokine production in the presence of the bacterial stimuli.
Figure 7
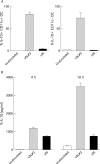
Probiotic bacteria (VSL#3) inhibit the generation of IFNγ-producing T cells. Naïve T cells and enriched DC treated with control medium, VSL#3 (at a dose equivalent to 108 CFU/ml) or LPS (1 μg/ml) were co-cultured. Cells were restimulated with PMA and ionomycin and cytokine production was assessed by intracellular cytokine staining. Staining for IFN-γ, IL-4, and IL-10 is shown. The proportion of cells and the geometric mean of fluorescence intensity are represented. Results are representative of four independent experiments.
Figure 8
Individual probiotic strains decrease IFN-γ production by polyclonally stimulated T cells. Individual probiotic strains at 108 CFU/ml were incubated with whole blood. Subsequently the cultures were stimulated with PMA and ionomycin and the effect of preculturing with the probiotic strain on cytokine production was observed. In some cultures an anti-IL-10 neutralising antibody or its isotype control were added and the effect on IFN-γ assessed. Results indicate the mean (SEM) of at least nine experiments.
Similar articles
- Effects of live and inactivated VSL#3 probiotic preparations in the modulation of in vitro and in vivo allergen-induced Th2 responses.
Mastrangeli G, Corinti S, Butteroni C, Afferni C, Bonura A, Boirivant M, Colombo P, Di Felice G. Mastrangeli G, et al. Int Arch Allergy Immunol. 2009;150(2):133-43. doi: 10.1159/000218116. Epub 2009 May 11. Int Arch Allergy Immunol. 2009. PMID: 19439979 - Crohn's disease intestinal CD4+ T cells have impaired interleukin-10 production which is not restored by probiotic bacteria.
Hvas CL, Kelsen J, Agnholt J, Höllsberg P, Tvede M, Møller JK, Dahlerup JF. Hvas CL, et al. Scand J Gastroenterol. 2007 May;42(5):592-601. doi: 10.1080/00365520601013754. Scand J Gastroenterol. 2007. PMID: 17454880 - Evidence for the role of interferon-alfa production by dendritic cells in the Th1 response in celiac disease.
Di Sabatino A, Pickard KM, Gordon JN, Salvati V, Mazzarella G, Beattie RM, Vossenkaemper A, Rovedatti L, Leakey NA, Croft NM, Troncone R, Corazza GR, Stagg AJ, Monteleone G, MacDonald TT. Di Sabatino A, et al. Gastroenterology. 2007 Oct;133(4):1175-87. doi: 10.1053/j.gastro.2007.08.018. Epub 2007 Aug 14. Gastroenterology. 2007. PMID: 17919493 - Probiotic modulation of dendritic cells and T cell responses in the intestine.
Meijerink M, Wells JM. Meijerink M, et al. Benef Microbes. 2010 Nov;1(4):317-26. doi: 10.3920/BM2010.0029. Benef Microbes. 2010. PMID: 21831770 Review. - Health-beneficial effects of probiotics: Its mode of action.
Ohashi Y, Ushida K. Ohashi Y, et al. Anim Sci J. 2009 Aug;80(4):361-71. doi: 10.1111/j.1740-0929.2009.00645.x. Anim Sci J. 2009. PMID: 20163595 Review.
Cited by
- The C-Type Lectin Receptor SIGNR3 Binds to Fungi Present in Commensal Microbiota and Influences Immune Regulation in Experimental Colitis.
Eriksson M, Johannssen T, von Smolinski D, Gruber AD, Seeberger PH, Lepenies B. Eriksson M, et al. Front Immunol. 2013 Jul 16;4:196. doi: 10.3389/fimmu.2013.00196. eCollection 2013. Front Immunol. 2013. PMID: 23882266 Free PMC article. - The gut microbiota in IBD.
Manichanh C, Borruel N, Casellas F, Guarner F. Manichanh C, et al. Nat Rev Gastroenterol Hepatol. 2012 Oct;9(10):599-608. doi: 10.1038/nrgastro.2012.152. Epub 2012 Aug 21. Nat Rev Gastroenterol Hepatol. 2012. PMID: 22907164 Review. - Probiotic Bio-Three induces Th1 and anti-inflammatory effects in PBMC and dendritic cells.
Hua MC, Lin TY, Lai MW, Kong MS, Chang HJ, Chen CC. Hua MC, et al. World J Gastroenterol. 2010 Jul 28;16(28):3529-40. doi: 10.3748/wjg.v16.i28.3529. World J Gastroenterol. 2010. PMID: 20653061 Free PMC article. - Does Probiotic Consumption Enhance Wound Healing? A Systematic Review.
Togo C, Zidorio AP, Gonçalves V, Botelho P, de Carvalho K, Dutra E. Togo C, et al. Nutrients. 2021 Dec 27;14(1):111. doi: 10.3390/nu14010111. Nutrients. 2021. PMID: 35010987 Free PMC article. - Epigenome targeting by probiotic metabolites.
Licciardi PV, Wong SS, Tang ML, Karagiannis TC. Licciardi PV, et al. Gut Pathog. 2010 Dec 21;2(1):24. doi: 10.1186/1757-4749-2-24. Gut Pathog. 2010. PMID: 21172038 Free PMC article.
References
- Akbari O , DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol 2001;2:725–31. - PubMed
- Williamson E , Bilsborough JM, Viney JL. Regulation of mucosal dendritic cell function by receptor activator of NF-kappa B (RANK)/RANK ligand interactions: impact on tolerance induction. J Immunol 2002;169:3606–12. - PubMed
- Viney JL, Mowat AM, O’Malley JM, et al. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol 1998;160:5815–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous