Estrogen deficiency and bone loss: an inflammatory tale - PubMed (original) (raw)
Review
Estrogen deficiency and bone loss: an inflammatory tale
M Neale Weitzmann et al. J Clin Invest. 2006 May.
Abstract
Estrogen plays a fundamental role in skeletal growth and bone homeostasis in both men and women. Although remarkable progress has been made in our understanding of how estrogen deficiency causes bone loss, the mechanisms involved have proven to be complex and multifaceted. Although estrogen is established to have direct effects on bone cells, recent animal studies have identified additional unexpected regulatory effects of estrogen centered at the level of the adaptive immune response. Furthermore, a potential role for reactive oxygen species has now been identified in both humans and animals. One major challenge is the integration of a multitude of redundant pathways and cytokines, each apparently capable of playing a relevant role, into a comprehensive model of postmenopausal osteoporosis. This Review presents our current understanding of the process of estrogen deficiency-mediated bone destruction and explores some recent findings and hypotheses to explain estrogen action in bone. Due to the inherent difficulties associated with human investigation, many of the lessons learned have been in animal models. Consequently, many of these principles await further validation in humans.
Figures
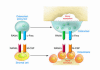
Figure 1. Cells and cytokines responsible for physiological OC renewal.
OC precursors may differentiate from the population of monocytes/macrophages, among which they circulate by virtue of their expression of the receptor RANK. When RANKL binds to this receptor in the presence of the trophic factor M-CSF, which in turn binds to its receptor, colony-stimulating factor receptor 1 (c-Fms), OC precursors differentiate and fuse together to form mature, multinucleated bone-resorbing OCs. Under physiological conditions the dominant source of RANKL and M-CSF in the bone marrow microenvironment is from the bone-forming cells, the OBs, and their SC precursors.
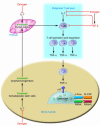
Figure 2. Estrogen suppresses T cell TNF production by regulating T cell differentiation and activity in the bone marrow, thymus, and peripheral lymphoid organs.
In the bone marrow, estrogen downregulates the proliferation of hematopoietic stem cells through an IL-7–dependent mechanism, resulting in a smaller pool of lymphoid progenitors. T cell precursors leave the bone marrow and migrate to the thymus, where T cell differentiation, selection, and expansion take place, in large measure under control of IL-7. Following release from the thymus (thymic output), these new T cells home to peripheral lymphoid organs, including the bone marrow itself. Estrogen prevents T cell activation in part by directly blunting antigen presentation and in part via repression of IL-7 and IFN-γ production. This effect is amplified by the upregulation of the IL-7 suppressor TGF-β. The net result of these actions is a decrease in the number of TNF-producing T cells. The blunted levels of TNF diminish RANKL-induced OC formation, ultimately preventing bone loss.
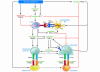
Figure 3. Schematic representation of the main mechanisms and feedback interactions by which estrogen deficiency leads to bone loss.
The bone loss induced by estrogen deficiency is due to a complex interplay of hormones and cytokines that converge to disrupt the process of bone remodeling. Estrogen deficiency leads to a global increase in IL-7 production in target organs such as bone, thymus, and spleen, in part through decreases in TGF-β and increased IGF-1 production. This leads to an initial wave of T cell activation. Activated T cells release IFN-γ, which increases antigen presentation by DCs and macrophages (Mϕ) by upregulating MHC class II expression through the transcription factor CIITA. Estrogen deficiency also amplifies T cell activation and osteoclastogenesis by downregulating antioxidant pathways, leading to an upswing in ROS. The resulting increase in ROS stimulates antigen presentation and the production of TNF by mature OCs. The combined effect of IFN-γ and ROS markedly enhances antigen presentation, amplifying T cell activation and promoting release of the osteoclastogenic factors RANKL and TNF. TNF further stimulates SC and OB RANKL and M-CSF production, in part via IL-1 upregulation, driving OC formation. TNF and IL-7 further exacerbate bone loss by blunting bone formation through direct repressive effects on OBs.
Similar articles
- Estrogen deficiency, T cells and bone loss.
Pacifici R. Pacifici R. Cell Immunol. 2008 Mar-Apr;252(1-2):68-80. doi: 10.1016/j.cellimm.2007.06.008. Epub 2007 Sep 20. Cell Immunol. 2008. PMID: 17888417 Review. - Are Oxidative Stress and Inflammation Mediators of Bone Loss Due to Estrogen Deficiency? A Review of Current Evidence.
Mohamad NV, Ima-Nirwana S, Chin KY. Mohamad NV, et al. Endocr Metab Immune Disord Drug Targets. 2020;20(9):1478-1487. doi: 10.2174/1871530320666200604160614. Endocr Metab Immune Disord Drug Targets. 2020. PMID: 32496996 Free PMC article. Review. - The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed?
Khosla S, Melton LJ 3rd, Riggs BL. Khosla S, et al. J Bone Miner Res. 2011 Mar;26(3):441-51. doi: 10.1002/jbmr.262. J Bone Miner Res. 2011. PMID: 20928874 Free PMC article. - BMI-1 Mediates Estrogen-Deficiency-Induced Bone Loss by Inhibiting Reactive Oxygen Species Accumulation and T Cell Activation.
Li J, Wang Q, Yang R, Zhang J, Li X, Zhou X, Miao D. Li J, et al. J Bone Miner Res. 2017 May;32(5):962-973. doi: 10.1002/jbmr.3059. Epub 2017 Jan 18. J Bone Miner Res. 2017. PMID: 27943387
Cited by
- Titanium Implant Osseointegration Problems with Alternate Solutions Using Epoxy/Carbon-Fiber-Reinforced Composite.
Petersen RC. Petersen RC. Metals (Basel). 2014 Dec;4(4):549-569. doi: 10.3390/met4040549. Metals (Basel). 2014. PMID: 25635227 Free PMC article. - Role of carotenoid β-cryptoxanthin in bone homeostasis.
Yamaguchi M. Yamaguchi M. J Biomed Sci. 2012 Apr 2;19(1):36. doi: 10.1186/1423-0127-19-36. J Biomed Sci. 2012. PMID: 22471523 Free PMC article. Review. - Exploratory analysis of the potential relationship between urinary molybdenum and bone mineral density among adult men and women from NHANES 2007-2010.
Lewis RC, Johns LE, Meeker JD. Lewis RC, et al. Chemosphere. 2016 Dec;164:677-682. doi: 10.1016/j.chemosphere.2016.08.142. Epub 2016 Sep 16. Chemosphere. 2016. PMID: 27639340 Free PMC article. - Polg mtDNA mutator mice reveal limited involvement of vertebral bone loss in premature aging-related thoracolumbar hyperkyphosis.
Roessinger O, Hügle T, Walker UA, Geurts J. Roessinger O, et al. Bone Rep. 2022 Aug 30;17:101618. doi: 10.1016/j.bonr.2022.101618. eCollection 2022 Dec. Bone Rep. 2022. PMID: 36120646 Free PMC article. - Association of chemerin levels and bone mineral density in Chinese obese postmenopausal women.
Shi L, Mao C, Wang X, Liu R, Li L, Mou X, Xu P, Li H, Xu C, Yuan G, Wan B, Zhang H. Shi L, et al. Medicine (Baltimore). 2016 Aug;95(35):e4583. doi: 10.1097/MD.0000000000004583. Medicine (Baltimore). 2016. PMID: 27583869 Free PMC article.
References
- Bouxsein M.L., et al. Ovariectomy-induced bone loss varies among inbred strains of mice. . J. Bone Miner. Res. 2005;20:1085–1092. - PubMed
- Komm B.S., et al. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science. 1988;241:81–84. - PubMed
- Tomkinson A., Gevers E.F., Wit J.M., Reeve J., Noble B.S. The role of estrogen in the control of rat osteocyte apoptosis. J. Bone Miner. Res. 1998;13:1243–1250. - PubMed
- Weitzmann M.N., Pacifici R. The role of T lymphocytes in bone metabolism. Immunol. Rev. 2005;208:154–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical