Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6 - PubMed (original) (raw)
Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6
Jaroslav Truksa et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Recently, it has been suggested that hepcidin, a peptide involved in iron homeostasis, is regulated by bone morphogenetic proteins (BMPs), apparently by binding to hemojuvelin (Hjv) as a coreceptor and signaling through Smad4. We investigate the role of Hfe, Tfr2 (transferrin receptor 2), and IL-6 in BMP2-, BMP4-, and BMP9-stimulated up-regulation of murine hepcidin, because these molecules, like Hjv, are known to be involved in hepcidin signaling. We show that the BMP signaling pathway acts independently of Hfe, Tfr2, and IL-6: The response to BMP2, BMP4, and BMP9 is similar in isolated hepatocytes of wild-type, Hfe(-/-), IL-6(-/-), and Tfr2(m) mutant mice. The potency of different human BMPs in stimulating hepcidin transcription by murine primary hepatocytes is BMP9 > BMP4 > BMP2. However, in human HepG2 cells, BMP4 and BMP9 are equally potent, whereas BMP2 requires a higher dose to become an effective hepcidin activator. Moreover, all of the tested BMPs are more potent regulators of hepcidin than IL-6 and thus are the most potent known stimulators of hepcidin transcription.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
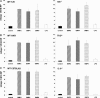
Effect of BMP2, BMP4, BMP9, and LPS treatment on hepcidin expression in hepatocytes isolated from _Hfe_−/−, _IL-6_−/−, and Tfr2 m mutant mice. Primary hepatocytes were isolated from perfused livers of _Hfe_−/− or wild-type 129 mice (A), Tfr2 m mutant or AKR wild-type mice (B), and _IL-_6−/− or C57BL/6J mice (C), seeded at 1.5 × 105 cells per well, and allowed to attach to collagen-coated 12-well plates for 2 h, and BMP2 (150 ng/ml), BMP4 (10 ng/ml), BMP9 (1 ng/ml), or LPS (200 ng/ml) was applied for 12–16 h. Hepcidin and S18 ribosomal protein (S18 RP) (a normalization gene) expression was assayed by quantitative real-time RT-PCR using TaqMan probes. All samples were processed in triplicate, and a graph of experimental values ± SEM from at least two independent experiments is shown.
Fig. 2.
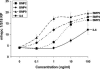
Dose–response curves of BMP2, BMP4, BMP9, and murine IL-6. Primary hepatocytes were isolated from perfused livers of C57BL/6J mice, seeded at 1.5 × 105 cells per well, allowed to attach to the collagen-coated 12-well plates, and treated with BMP2, BMP4, BMP9, and murine IL-6 at concentrations ranging from 0.1 to 100 ng/ml for 12–16 h. Hepcidin and 18S ribosomal protein (S18 RP) (a normalization gene) expression was assayed by quantitative real-time RT-PCR using TaqMan probes. All samples were processed in triplicate, and the mean and SEM are shown.
Fig. 3.
Effect of BMP2, BMP4, and BMP9 treatment on hepcidin expression in human HepG2 cells. (A) Human HepG2 hepatoma cells were seeded at 1.5 × 105 cells per well and allowed to attach to 12-well plates, and BMP2 (150 ng/ml), BMP4 (10 ng/ml), or BMP9 (1 ng/ml) was added. After 12–16 h of incubation, human hepcidin and human RPLP2 (a normalization gene) expression was assayed by quantitative real-time RT-PCR using TaqMan probes. All samples were processed in triplicate, and a graph of experimental values ± SEM from three independent experiments is shown. (B) Dose–response curves of BMP2, BMP4, BMP9, and human IL-6. Human hepatoma cells (HepG2) were seeded at 1.5 × 105 cells per well, allowed to attach to 12-well plates, and treated with BMP2, BMP4, BMP9, and human IL-6 at concentrations ranging from 0.1 to 100 ng/ml for 12–16 h. Human hepcidin and RPLP2 expression was assayed by quantitative real-time RT-PCR using TaqMan probes. All samples were processed in triplicate, and the mean and SEM are shown.
Similar articles
- The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation.
D'Alessio F, Hentze MW, Muckenthaler MU. D'Alessio F, et al. J Hepatol. 2012 Nov;57(5):1052-60. doi: 10.1016/j.jhep.2012.06.015. Epub 2012 Jun 21. J Hepatol. 2012. PMID: 22728873 - Iron regulation of hepcidin despite attenuated Smad1,5,8 signaling in mice without transferrin receptor 2 or Hfe.
Corradini E, Rozier M, Meynard D, Odhiambo A, Lin HY, Feng Q, Migas MC, Britton RS, Babitt JL, Fleming RE. Corradini E, et al. Gastroenterology. 2011 Nov;141(5):1907-14. doi: 10.1053/j.gastro.2011.06.077. Epub 2011 Jul 13. Gastroenterology. 2011. PMID: 21745449 Free PMC article. - Differing impact of the deletion of hemochromatosis-associated molecules HFE and transferrin receptor-2 on the iron phenotype of mice lacking bone morphogenetic protein 6 or hemojuvelin.
Latour C, Besson-Fournier C, Meynard D, Silvestri L, Gourbeyre O, Aguilar-Martinez P, Schmidt PJ, Fleming MD, Roth MP, Coppin H. Latour C, et al. Hepatology. 2016 Jan;63(1):126-37. doi: 10.1002/hep.28254. Epub 2015 Nov 12. Hepatology. 2016. PMID: 26406355 - The molecular pathogenesis of hereditary hemochromatosis.
Babitt JL, Lin HY. Babitt JL, et al. Semin Liver Dis. 2011 Aug;31(3):280-92. doi: 10.1055/s-0031-1286059. Epub 2011 Sep 7. Semin Liver Dis. 2011. PMID: 21901658 Review. - [Pathophysiology and genetics of classic HFE (type 1) hemochromatosis].
Loréal O, Ropert M, Mosser A, Déhais V, Deugnier Y, David V, Brissot P, Jouanolle AM. Loréal O, et al. Presse Med. 2007 Sep;36(9 Pt 2):1271-7. doi: 10.1016/j.lpm.2007.03.038. Epub 2007 May 22. Presse Med. 2007. PMID: 17521857 Review. French.
Cited by
- Alcohol Activates TGF-Beta but Inhibits BMP Receptor-Mediated Smad Signaling and Smad4 Binding to Hepcidin Promoter in the Liver.
Gerjevic LN, Liu N, Lu S, Harrison-Findik DD. Gerjevic LN, et al. Int J Hepatol. 2012;2012:459278. doi: 10.1155/2012/459278. Epub 2011 Oct 23. Int J Hepatol. 2012. PMID: 22121494 Free PMC article. - Bone morphogenetic protein-9 induces osteogenic differentiation of rat dental follicle stem cells in P38 and ERK1/2 MAPK dependent manner.
Li C, Yang X, He Y, Ye G, Li X, Zhang X, Zhou L, Deng F. Li C, et al. Int J Med Sci. 2012;9(10):862-71. doi: 10.7150/ijms.5027. Epub 2012 Nov 5. Int J Med Sci. 2012. PMID: 23155360 Free PMC article. - Hepcidin-ferroportin axis in health and disease.
Ginzburg YZ. Ginzburg YZ. Vitam Horm. 2019;110:17-45. doi: 10.1016/bs.vh.2019.01.002. Epub 2019 Feb 8. Vitam Horm. 2019. PMID: 30798811 Free PMC article. Review. - BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling.
Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, Su YX, Jiang W, Tang M, He Y, Wang Y, Chen L, Zuo GW, Shen J, Pan X, Reid RR, Luu HH, Haydon RC, He TC. Tang N, et al. J Cell Mol Med. 2009 Aug;13(8B):2448-2464. doi: 10.1111/j.1582-4934.2008.00569.x. Epub 2008 Nov 3. J Cell Mol Med. 2009. PMID: 19175684 Free PMC article.
References
- Varga A. C., Wrana J. L. Oncogene. 2005;24:5713–5721. - PubMed
- Gambaro K., Aberdam E., Virolle T., Aberdam D., Rouleau M. Cell Death Differ. 2006;13:1075–1087. - PubMed
- Ten D. P., Korchynskyi O., Valdimarsdottir G., Goumans M. J. Mol. Cell. Endocrinol. 2003;211:105–113. - PubMed
- Ten D. P., Hill C. S. Trends Biochem. Sci. 2004;29:265–273. - PubMed
- Liu F. Front. Biosci. 2003;8:s1280–s1303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous