Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer - PubMed (original) (raw)
Review
Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer
Celeste M Nelson et al. Annu Rev Cell Dev Biol. 2006.
Abstract
The microenvironment influences gene expression so that the behavior of a cell is largely determined by its interactions with the extracellular matrix, neighboring cells, and soluble local and systemic cues. We describe the essential roles of context and organ structure in directing mammary gland development and differentiated function and in determining the response to oncogenic insults, including mutations. We expand on the concept of "dynamic reciprocity" to present an integrated view of development, cancer, and aging and posit that genes are like the keys on a piano: Although they are essential, it is the context that makes the music.
Figures
Figure 1
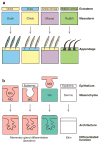
(a) The original model of dynamic reciprocity, or the minimum required unit for tissue-specific functions. N, nucleus; MT, microtubules; IF, intermediate filaments; MF, microfilaments; C, collagen. Reprinted from Bissell et al. (1982) with permission from Elsevier. (b) A more complete view of dynamic reciprocity.
Figure 2
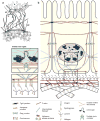
The dramatic effect of tissue-tissue interactions. (a) Embryonic ectoderm/mesoderm recombination experiments determined that the identity of the mesoderm dictated the identity of the ectodermal appendage. (b) Epithelial/mesenchymal recombination experiments determined that the identity of the mesenchyme dictated the architecture of the developing epithelium. When mammary gland (MG) epithelium is recombined with salivary gland (SG) mesenchyme, the resulting structure can still produce milk, although the epithelial tree resembles a salivary gland. Panel b adapted from Parmar & Cunha (2004).
Figure 3
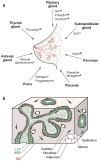
The structure and function of the mammary gland are influenced by communication with distant organs and between constituent tissues. (a) The human breast is a bilayered epithelial ductal tree (pink) embedded in a complex stroma. Signals released from distant organs influence ductal and acinar morphogenesis during puberty (*) and pregnancy (#) (reviewed in Hovey et al. 2002). (b) The epithelium consists of a layer of luminal epithelial cells (LEP) surrounded by myoepithelial cells (MEP) and basement membrane (BM). The epithelium is surrounded by a fibrous stromal compartment and adjacent fatty stroma. Molecular details of epithelial-mesenchymal interactions are described in Table 1.
Similar articles
- Integrated extracellular matrix signaling in mammary gland development and breast cancer progression.
Zhu J, Xiong G, Trinkle C, Xu R. Zhu J, et al. Histol Histopathol. 2014 Sep;29(9):1083-92. doi: 10.14670/HH-29.1083. Epub 2014 Mar 28. Histol Histopathol. 2014. PMID: 24682974 Free PMC article. Review. - Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices.
Xu R, Boudreau A, Bissell MJ. Xu R, et al. Cancer Metastasis Rev. 2009 Jun;28(1-2):167-76. doi: 10.1007/s10555-008-9178-z. Cancer Metastasis Rev. 2009. PMID: 19160017 Free PMC article. Review. - Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging.
Ghajar CM, Bissell MJ. Ghajar CM, et al. Histochem Cell Biol. 2008 Dec;130(6):1105-18. doi: 10.1007/s00418-008-0537-1. Epub 2008 Nov 14. Histochem Cell Biol. 2008. PMID: 19009245 Free PMC article. Review. - Cell-matrix interactions in mammary gland development and breast cancer.
Muschler J, Streuli CH. Muschler J, et al. Cold Spring Harb Perspect Biol. 2010 Oct;2(10):a003202. doi: 10.1101/cshperspect.a003202. Epub 2010 Aug 11. Cold Spring Harb Perspect Biol. 2010. PMID: 20702598 Free PMC article. Review. - On cell-matrix interactions in mammary gland development and breast cancer.
Talhouk R. Talhouk R. Cold Spring Harb Perspect Biol. 2012 Aug 1;4(8):a013540. doi: 10.1101/cshperspect.a013540. Cold Spring Harb Perspect Biol. 2012. PMID: 22855728 Free PMC article. Review.
Cited by
- Calcium-Oxidant Signaling Network Regulates AMP-activated Protein Kinase (AMPK) Activation upon Matrix Deprivation.
Sundararaman A, Amirtham U, Rangarajan A. Sundararaman A, et al. J Biol Chem. 2016 Jul 8;291(28):14410-29. doi: 10.1074/jbc.M116.731257. Epub 2016 May 11. J Biol Chem. 2016. PMID: 27226623 Free PMC article. - Hyperacetylation of Microtubules in Mesenchymal Cells Increases Cytokeratin 14-Positive Epithelial Progenitors in Developing Salivary Glands.
Joo EE, Lombaert IM, Yamada KM. Joo EE, et al. J Dent Res. 2016 Dec;95(13):1518-1527. doi: 10.1177/0022034516662450. Epub 2016 Aug 19. J Dent Res. 2016. PMID: 27542391 Free PMC article. - Personalized Medicine Approaches in Prostate Cancer Employing Patient Derived 3D Organoids and Humanized Mice.
Bartucci M, Ferrari AC, Kim IY, Ploss A, Yarmush M, Sabaawy HE. Bartucci M, et al. Front Cell Dev Biol. 2016 Jun 23;4:64. doi: 10.3389/fcell.2016.00064. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27446916 Free PMC article. - In vitro microvessels for the study of angiogenesis and thrombosis.
Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, López JA, Stroock AD. Zheng Y, et al. Proc Natl Acad Sci U S A. 2012 Jun 12;109(24):9342-7. doi: 10.1073/pnas.1201240109. Epub 2012 May 29. Proc Natl Acad Sci U S A. 2012. PMID: 22645376 Free PMC article. - Phosphoinositide 3-kinase p110δ promotes lumen formation through the enhancement of apico-basal polarity and basal membrane organization.
Peng J, Awad A, Sar S, Komaiha OH, Moyano R, Rayal A, Samuel D, Shewan A, Vanhaesebroeck B, Mostov K, Gassama-Diagne A. Peng J, et al. Nat Commun. 2015 Jan 13;6:5937. doi: 10.1038/ncomms6937. Nat Commun. 2015. PMID: 25583025 Free PMC article.
References
- Aggeler J, Ward J, Blackie LM, Barcellos-Hoff MH, Streuli CH, Bissell MJ. Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo. J Cell Sci. 1991;99(Pt. 2):407–17. - PubMed
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. - PubMed
Publication types
MeSH terms
Grants and funding
- R37 CA064786/CA/NCI NIH HHS/United States
- R01 CA064786-06/CA/NCI NIH HHS/United States
- R01 CA057621/CA/NCI NIH HHS/United States
- CA64786/CA/NCI NIH HHS/United States
- CA57621/CA/NCI NIH HHS/United States
- R01 CA057621-07/CA/NCI NIH HHS/United States
- R01 CA064786/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical