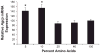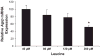Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism - PubMed (original) (raw)
Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism
Christopher D Morrison et al. Am J Physiol Endocrinol Metab. 2007 Jul.
Abstract
Metabolic fuels act on hypothalamic neurons to regulate feeding behavior and energy homeostasis, but the signaling mechanisms mediating these effects are not fully clear. Rats placed on a low-protein diet (10% of calories) exhibited increased food intake (P < 0.05) and hypothalamic Agouti-related protein (Agrp) gene expression (P = 0.002). Direct intracerebroventricular injection of either an amino acid mixture (RPMI 1640) or leucine alone (1 mug) suppressed 24-h food intake (P < 0.05), indicating that increasing amino acid concentrations within the brain is sufficient to suppress food intake. To define a cellular mechanism for these direct effects, GT1-7 hypothalamic cells were exposed to low amino acids for 16 h. Decreasing amino acid availability increased Agrp mRNA levels in GT1-7 cells (P < 0.01), and this effect was attenuated by replacement of the amino acid leucine (P < 0.05). Acute exposure to elevated amino acid concentrations increased ribosomal protein S6 kinase phosphorylation via a rapamycin-sensitive mechanism, suggesting that amino acids directly stimulated mammalian target of rapamycin (mTOR) signaling. To test whether mTOR signaling contributes to amino acid inhibition of Agrp gene expression, GT1-7 cells cultured in either low or high amino acids for 16 h and were also treated with rapamcyin (50 nM). Rapamycin treatment increased Agrp mRNA levels in cells exposed to high amino acids (P = 0.01). Taken together, these observations indicate that amino acids can act within the brain to inhibit food intake and that a direct, mTOR-dependent inhibition of Agrp gene expression may contribute to this effect.
Figures
Fig. 1
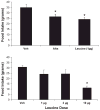
Effects of a low-protein diet on food intake. Male Sprague Dawley rats were placed on a purified, isocaloric diet providing protein at either 20% of total energy (20% protein; control) or 10% of total energy (7–9 rats/group), and changes in food intake and body weight were assessed over 7 days. Exposure to the low-protein diet resulted in a significant increase in daily food intake beginning on day 3, and this increase persisted for the remainder of the study (*P < 0.05).
Fig. 2
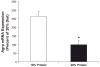
Effects of a low-protein diet on hypothalamic Agrp gene expression. After 7 days on the low-protein diet, brains were collected, small punches of mediobasal hypothalamus were collected, and total RNA was extracted. Levels of Agrp mRNA were assessed via real-time PCR. Hypothalamic Agrp mRNA expression was significantly increased in rats exposed to 7 days of a low-protein diet compared with controls (*P = 0.002).
Fig. 3
Effect of acute, central administration of amino acids on food intake. A: rats bearing 3rd-ventricular cannulas were fasted for 24 h and subsequently injected (icv; 7 rats/group) with vehicle, 1 μg of leucine, or a cell culture-based amino acid mixture (10× RPMI; 2 μl) that contained 1 μg of leucine in addition to other essential amino acids. Injection of both the amino acid mixture and leucine significantly suppressed 4- and 24-h food intake (*P < 0.05). B: a separate group of icv-cannulated rats was fasted as above and injected icv with increasing doses of leucine (1, 3, and 10 μg; 7–9 rats/group), and food intake during the subsequent 24 h was determined. Icv injection of leucine dose-dependently suppressed 4 and 24-h food intake, with 1 μg leucine exerting a modest anorectic effect at 24 h (P = 0.06) and 10 μg leucine robustly suppressing intake (*P < 0.01).
Fig. 4
Effects of reduced amino acid availability on Agrp gene expression in vitro. The GT1–7 hypothalamic cell line was used to assess the effects of reduced amino acids on Agrp gene expression in vitro. Cells were cultured in 5, 10, 25, 40, and 100% amino acids for 16 h, with 100% representing standard DMEM. Following exposure to low amino acids, cells were harvested to assess Agrp gene expression via real-time PCR. Decreasing amino acid levels to 10 or 5% of basal resulted in significant increases in Agrp mRNA levels (*P < 0.001).
Fig. 5
Effect of leucine on Agrp gene expression in vitro. GT1–7 cells were cultured in 5% amino acids for 16 h and supplemented with 40, 80, 120, or 200 μM leucine. After 16 h, cells were harvested, total RNA was extracted, and Agrp mRNA levels were determined via real-time PCR. Leucine supplementation to cells cultured in low amino acids resulted in a dose-dependent, linear decrease in Agrp mRNA levels in cells cultured in low amino acid conditions (P < 0.01).
Fig. 6
Effect of amino acids and insulin on mammalian target of rapamycin (mTOR) signaling in hypothalamic cells. A: GT1–7 cells were cultured at 5% of basal amino acid concentrations for 7 h and then placed in media containing 100% amino acids. At defined time points following exposure to high amino acids, cells were collected for Western blot analysis of ribosomal protein S6 kinase (S6K1) phosphorylation. B: in similar fashion, GT1–7 cells cultured in normal levels of amino acids were treated with insulin (10 mM), cells were collected at similar time points, and total protein was used to assess phosphorylation of S6K1. C: to test whether amino acid-dependent activation of S6K1 requires mTOR signaling, GT1–7 cells incubated in low amino acid concentrations were treated for 20 min with either 100% amino acids or insulin (10 mM) in the presence or absence of the mTOR inhibitor rapamycin. Rapamycin treatment (50 nM) attenuated both amino acid- and insulin-induced increases in S6K1 phosphorylation (*P < 0.05).
Fig. 7
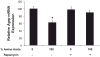
Activation of mTOR is necessary for amino acid-dependent inhibition of Agrp gene expression. GT1–7 cells were cultured in either 5 or 100% amino acids in the presence or absence of rapamycin (50 nm), generating four treatment groups. After 16 h of culture, cells were harvested, and RNA was extracted to assess Agrp gene expression via real-time PCR. Treatment with rapamycin had no effect on the already elevated Agrp mRNA levels in cells cultured in 5% amino acids but increased Agrp mRNA levels in cells cultured in 100% amino acids such that levels were significantly higher than those of cells cultured in 100% amino acids without rapamycin (*P = 0.01).
Similar articles
- Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling.
Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Morrison CD, et al. Am J Physiol Endocrinol Metab. 2005 Dec;289(6):E1051-7. doi: 10.1152/ajpendo.00094.2005. Epub 2005 Jul 26. Am J Physiol Endocrinol Metab. 2005. PMID: 16046456 - The ketone body β-hydroxybutyric acid influences agouti-related peptide expression via AMP-activated protein kinase in hypothalamic GT1-7 cells.
Laeger T, Pöhland R, Metges CC, Kuhla B. Laeger T, et al. J Endocrinol. 2012 May;213(2):193-203. doi: 10.1530/JOE-11-0457. Epub 2012 Feb 22. J Endocrinol. 2012. PMID: 22357971 - Linoleic acid and stearic acid elicit opposite effects on AgRP expression and secretion via TLR4-dependent signaling pathways in immortalized hypothalamic N38 cells.
Wang S, Xiang N, Yang L, Zhu C, Zhu X, Wang L, Gao P, Xi Q, Zhang Y, Shu G, Jiang Q. Wang S, et al. Biochem Biophys Res Commun. 2016 Mar 18;471(4):566-71. doi: 10.1016/j.bbrc.2016.02.031. Epub 2016 Feb 12. Biochem Biophys Res Commun. 2016. PMID: 26879142 - Role of mTOR signalling in the control of translation initiation and elongation by nutrients.
Proud CG. Proud CG. Curr Top Microbiol Immunol. 2004;279:215-44. doi: 10.1007/978-3-642-18930-2_13. Curr Top Microbiol Immunol. 2004. PMID: 14560960 Review. - Amino acids as regulators of gene expression at the level of mRNA translation.
Jefferson LS, Kimball SR. Jefferson LS, et al. J Nutr. 2003 Jun;133(6 Suppl 1):2046S-2051S. doi: 10.1093/jn/133.6.2046S. J Nutr. 2003. PMID: 12771363 Review.
Cited by
- mTORC1 Signaling in AgRP Neurons Is Not Required to Induce Major Neuroendocrine Adaptations to Food Restriction.
de Souza GO, Teixeira PDS, Câmara NOS, Donato J Jr. de Souza GO, et al. Cells. 2023 Oct 12;12(20):2442. doi: 10.3390/cells12202442. Cells. 2023. PMID: 37887286 Free PMC article. - Protein appetite as an integrator in the obesity system: the protein leverage hypothesis.
Raubenheimer D, Simpson SJ. Raubenheimer D, et al. Philos Trans R Soc Lond B Biol Sci. 2023 Oct 23;378(1888):20220212. doi: 10.1098/rstb.2022.0212. Epub 2023 Sep 4. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37661737 Free PMC article. Review. - l-Type amino acid transporter 1 in hypothalamic neurons in mice maintains energy and bone homeostasis.
Park G, Fukasawa K, Horie T, Masuo Y, Inaba Y, Tatsuno T, Yamada T, Tokumura K, Iwahashi S, Iezaki T, Kaneda K, Kato Y, Ishigaki Y, Mieda M, Tanaka T, Ogawa K, Ochi H, Sato S, Shi YB, Inoue H, Lee H, Hinoi E. Park G, et al. JCI Insight. 2023 Apr 10;8(7):e154925. doi: 10.1172/jci.insight.154925. JCI Insight. 2023. PMID: 36862514 Free PMC article. - Agouti-related protein as the glucose signaling sensor in the central melanocortin circuits in regulating fish food intake.
Han J, Liang X, Guo Y, Wu X, Li Z, Hong T. Han J, et al. Front Endocrinol (Lausanne). 2022 Nov 1;13:1010472. doi: 10.3389/fendo.2022.1010472. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36387900 Free PMC article. Review. - Protein Appetite at the Interface between Nutrient Sensing and Physiological Homeostasis.
Khan MS, Spann RA, Münzberg H, Yu S, Albaugh VL, He Y, Berthoud HR, Morrison CD. Khan MS, et al. Nutrients. 2021 Nov 16;13(11):4103. doi: 10.3390/nu13114103. Nutrients. 2021. PMID: 34836357 Free PMC article. Review.
References
- Anderson GH, Moore SE. Dietary proteins in the regulation of food intake and body weight in humans. J Nutr. 2004;134:974S–979S. - PubMed
- Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. - PubMed
- Bensaid A, Tome D, L’Heureux-Bourdon D, Even P, Gietzen D, Morens C, Gaudichon C, Larue-Achagiotis C, Fromentin G. A high-protein diet enhances satiety without conditioned taste aversion in the rat. Physiol Behav. 2003;78:311–320. - PubMed
- Choi YH, Fletcher PJ, Anderson GH. Extracellular amino acid profiles in the paraventricular nucleus of the rat hypothalamus are influenced by diet composition. Brain Res. 2001;892:320–328. - PubMed
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RR-021945/RR/NCRR NIH HHS/United States
- P20 RR021945/RR/NCRR NIH HHS/United States
- P30 DK072476/DK/NIDDK NIH HHS/United States
- NS-051570/NS/NINDS NIH HHS/United States
- R03 NS051570/NS/NINDS NIH HHS/United States
- P20 RR021945-027777/RR/NCRR NIH HHS/United States
- P20 GM103528/GM/NIGMS NIH HHS/United States
- P30 GM118430/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous