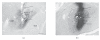Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test - PubMed (original) (raw)
Comparative Study
Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test
Gabor Legradi et al. Neural Plast. 2007.
Abstract
High concentrations of pituitary adenylate cyclase-activating polypeptide (PACAP) nerve fibers are present in the central nucleus of amygdala (CeA), a brain region implicated in the control of fear-related behavior. This study evaluated PACAPergic modulation of fear responses at the CeA in male Sprague-Dawley rats. PACAP (50-100 pmol) microinfusion via intra-CeA cannulae produced increases in immobility and time the rats spent withdrawn into a corner opposite to the electrified probe compared to controls in the shock-probe fear/defensive burying test. Shock-probe burying and exploration, numbers of shocks received, locomotion distance, and velocity were all reduced by intra-CeA PACAP injection. Further, intra-CeA PACAP effects were manifested only when the animals were challenged by shock, as intra-CeA PACAP injections did not cause significant changes in the behaviors of unshocked rats. Thus, intra-CeA administration of PACAP produces a distinct reorganization of stress-coping behaviors from active (burying) to passive modes, such as withdrawal and immobility. These findings are potentially significant toward enhancing our understanding of the involvement of PACAP and the CeA in the neural basis of fear and anxiety.
Figures
Figure 1
Histological verification of an injection site produced by microinjected synthetic PACAP. (a) Section from control brain, injected with aCSF vehicle. (b) Injected synthetic PACAP (50 pmol) immunoreactivity in the CeA. BLA = basolateral nucleus of the amygdala, CeM = central nucleus of the amygdala medial part, CeL = central nucleus of the amygdala, lateral part. Note the presence of high density of endogenous PACAP fibers in both (a) and (b). Arrows indicate the location of cannula track. In (b), synthetic PACAP injection is visible as an intense dark reaction product. Scale bar = 200 _μ_m.
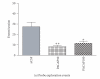
Figure 2
Effects of intra-CeA microinfusion of PACAP on shock-probe exploration and zonal preference in shocked rats. The numbers of probe exploration events (a) and time spent on probe exploration (b) are significantly reduced by intra-CeA PACAP. Zonal preference is altered by intra-CeA PACAP microinjection as rats spent significantly less time in the near zone (c) but more time in the zone away from the electrified shock probe (d). *P < .05 and **P ≤ .001 compared to aCSF controls. (aCSF n = 7/group, PACAP50 n = 7/group, PACAP100 n = 4/group).
Figure 2
Effects of intra-CeA microinfusion of PACAP on shock-probe exploration and zonal preference in shocked rats. The numbers of probe exploration events (a) and time spent on probe exploration (b) are significantly reduced by intra-CeA PACAP. Zonal preference is altered by intra-CeA PACAP microinjection as rats spent significantly less time in the near zone (c) but more time in the zone away from the electrified shock probe (d). *P < .05 and **P ≤ .001 compared to aCSF controls. (aCSF n = 7/group, PACAP50 n = 7/group, PACAP100 n = 4/group).
Figure 2
Effects of intra-CeA microinfusion of PACAP on shock-probe exploration and zonal preference in shocked rats. The numbers of probe exploration events (a) and time spent on probe exploration (b) are significantly reduced by intra-CeA PACAP. Zonal preference is altered by intra-CeA PACAP microinjection as rats spent significantly less time in the near zone (c) but more time in the zone away from the electrified shock probe (d). *P < .05 and **P ≤ .001 compared to aCSF controls. (aCSF n = 7/group, PACAP50 n = 7/group, PACAP100 n = 4/group).
Figure 2
Effects of intra-CeA microinfusion of PACAP on shock-probe exploration and zonal preference in shocked rats. The numbers of probe exploration events (a) and time spent on probe exploration (b) are significantly reduced by intra-CeA PACAP. Zonal preference is altered by intra-CeA PACAP microinjection as rats spent significantly less time in the near zone (c) but more time in the zone away from the electrified shock probe (d). *P < .05 and **P ≤ .001 compared to aCSF controls. (aCSF n = 7/group, PACAP50 n = 7/group, PACAP100 n = 4/group).
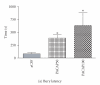
Figure 3
Effects of intra-CeA PACAP microinjection on locomotion parameters in shocked rats. Total distance moved (a) and mean movement velocity (b) were significantly reduced by intra-CeA PACAP microinjection. Immobility events (c) and total time spent on immobility (d) following probe-contact-induced shocks were increased by intra-CeA PACAP. *P < .05 and **P ≤ .001 compared to aCSF controls.
Figure 3
Effects of intra-CeA PACAP microinjection on locomotion parameters in shocked rats. Total distance moved (a) and mean movement velocity (b) were significantly reduced by intra-CeA PACAP microinjection. Immobility events (c) and total time spent on immobility (d) following probe-contact-induced shocks were increased by intra-CeA PACAP. *P < .05 and **P ≤ .001 compared to aCSF controls.
Figure 3
Effects of intra-CeA PACAP microinjection on locomotion parameters in shocked rats. Total distance moved (a) and mean movement velocity (b) were significantly reduced by intra-CeA PACAP microinjection. Immobility events (c) and total time spent on immobility (d) following probe-contact-induced shocks were increased by intra-CeA PACAP. *P < .05 and **P ≤ .001 compared to aCSF controls.
Figure 3
Effects of intra-CeA PACAP microinjection on locomotion parameters in shocked rats. Total distance moved (a) and mean movement velocity (b) were significantly reduced by intra-CeA PACAP microinjection. Immobility events (c) and total time spent on immobility (d) following probe-contact-induced shocks were increased by intra-CeA PACAP. *P < .05 and **P ≤ .001 compared to aCSF controls.
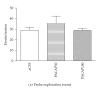
Figure 4
Effect of intra-CeA PACAP administration on shock-probe burying and shock-related behaviors. Latency to bury the electrified shock probe (a) was significantly increased in PACAP-injected animals whereas burying events (b) and time (c) and the height of bedding over the probe (d) were reduced. The number of probe-contact-induced shocks (e) was significantly reduced in PACAP-injected animals but shock reactivity (e) was unaltered. *P < .05 and **P ≤ .001 compared to aCSF controls.
Figure 4
Effect of intra-CeA PACAP administration on shock-probe burying and shock-related behaviors. Latency to bury the electrified shock probe (a) was significantly increased in PACAP-injected animals whereas burying events (b) and time (c) and the height of bedding over the probe (d) were reduced. The number of probe-contact-induced shocks (e) was significantly reduced in PACAP-injected animals but shock reactivity (e) was unaltered. *P < .05 and **P ≤ .001 compared to aCSF controls.
Figure 4
Effect of intra-CeA PACAP administration on shock-probe burying and shock-related behaviors. Latency to bury the electrified shock probe (a) was significantly increased in PACAP-injected animals whereas burying events (b) and time (c) and the height of bedding over the probe (d) were reduced. The number of probe-contact-induced shocks (e) was significantly reduced in PACAP-injected animals but shock reactivity (e) was unaltered. *P < .05 and **P ≤ .001 compared to aCSF controls.
Figure 4
Effect of intra-CeA PACAP administration on shock-probe burying and shock-related behaviors. Latency to bury the electrified shock probe (a) was significantly increased in PACAP-injected animals whereas burying events (b) and time (c) and the height of bedding over the probe (d) were reduced. The number of probe-contact-induced shocks (e) was significantly reduced in PACAP-injected animals but shock reactivity (e) was unaltered. *P < .05 and **P ≤ .001 compared to aCSF controls.
Figure 4
Effect of intra-CeA PACAP administration on shock-probe burying and shock-related behaviors. Latency to bury the electrified shock probe (a) was significantly increased in PACAP-injected animals whereas burying events (b) and time (c) and the height of bedding over the probe (d) were reduced. The number of probe-contact-induced shocks (e) was significantly reduced in PACAP-injected animals but shock reactivity (e) was unaltered. *P < .05 and **P ≤ .001 compared to aCSF controls.
Figure 4
Effect of intra-CeA PACAP administration on shock-probe burying and shock-related behaviors. Latency to bury the electrified shock probe (a) was significantly increased in PACAP-injected animals whereas burying events (b) and time (c) and the height of bedding over the probe (d) were reduced. The number of probe-contact-induced shocks (e) was significantly reduced in PACAP-injected animals but shock reactivity (e) was unaltered. *P < .05 and **P ≤ .001 compared to aCSF controls.
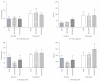
Figure 5
Summary of relevant behaviors of rats tested with the unelectrified shock probe (unshocked groups). Intra-CeA PACAP microinjection, in the absence of shocks, had no significant main effect on rat behaviors in the test chamber. (a) probe exploration events, (b) probe exploration time, (c) near zone time, (d) away zone time, (e) total distance moved, and (f) movement velocity. *P < .05 and **P ≤ .001 compared to aCSF controls. (aCSF n = 6/group, PACAP50 n = 4/group, PACAP100 n = 5/group.)
Figure 5
Summary of relevant behaviors of rats tested with the unelectrified shock probe (unshocked groups). Intra-CeA PACAP microinjection, in the absence of shocks, had no significant main effect on rat behaviors in the test chamber. (a) probe exploration events, (b) probe exploration time, (c) near zone time, (d) away zone time, (e) total distance moved, and (f) movement velocity. *P < .05 and **P ≤ .001 compared to aCSF controls. (aCSF n = 6/group, PACAP50 n = 4/group, PACAP100 n = 5/group.)
Figure 5
Summary of relevant behaviors of rats tested with the unelectrified shock probe (unshocked groups). Intra-CeA PACAP microinjection, in the absence of shocks, had no significant main effect on rat behaviors in the test chamber. (a) probe exploration events, (b) probe exploration time, (c) near zone time, (d) away zone time, (e) total distance moved, and (f) movement velocity. *P < .05 and **P ≤ .001 compared to aCSF controls. (aCSF n = 6/group, PACAP50 n = 4/group, PACAP100 n = 5/group.)
Figure 5
Summary of relevant behaviors of rats tested with the unelectrified shock probe (unshocked groups). Intra-CeA PACAP microinjection, in the absence of shocks, had no significant main effect on rat behaviors in the test chamber. (a) probe exploration events, (b) probe exploration time, (c) near zone time, (d) away zone time, (e) total distance moved, and (f) movement velocity. *P < .05 and **P ≤ .001 compared to aCSF controls. (aCSF n = 6/group, PACAP50 n = 4/group, PACAP100 n = 5/group.)
Figure 5
Summary of relevant behaviors of rats tested with the unelectrified shock probe (unshocked groups). Intra-CeA PACAP microinjection, in the absence of shocks, had no significant main effect on rat behaviors in the test chamber. (a) probe exploration events, (b) probe exploration time, (c) near zone time, (d) away zone time, (e) total distance moved, and (f) movement velocity. *P < .05 and **P ≤ .001 compared to aCSF controls. (aCSF n = 6/group, PACAP50 n = 4/group, PACAP100 n = 5/group.)
Figure 5
Summary of relevant behaviors of rats tested with the unelectrified shock probe (unshocked groups). Intra-CeA PACAP microinjection, in the absence of shocks, had no significant main effect on rat behaviors in the test chamber. (a) probe exploration events, (b) probe exploration time, (c) near zone time, (d) away zone time, (e) total distance moved, and (f) movement velocity. *P < .05 and **P ≤ .001 compared to aCSF controls. (aCSF n = 6/group, PACAP50 n = 4/group, PACAP100 n = 5/group.)
Figure 6
Grooming and rearing behaviors of shocked and unshocked rats in the shock-probe fear chamber. Intra-CeA PACAP microinjection had no statistically significant effects on grooming events (a) and time (b) or rearing events (c) and time (d) in shocked or unshocked groups of rats, relative to their respective aCSF-injected controls.
Similar articles
- PACAP increases Arc/Arg 3.1 expression within the extended amygdala after fear conditioning in rats.
Meloni EG, Kaye KT, Venkataraman A, Carlezon WA Jr. Meloni EG, et al. Neurobiol Learn Mem. 2019 Jan;157:24-34. doi: 10.1016/j.nlm.2018.11.011. Epub 2018 Nov 17. Neurobiol Learn Mem. 2019. PMID: 30458282 Free PMC article. - Parabrachial Pituitary Adenylate Cyclase-Activating Polypeptide Activation of Amygdala Endosomal Extracellular Signal-Regulated Kinase Signaling Regulates the Emotional Component of Pain.
Missig G, Mei L, Vizzard MA, Braas KM, Waschek JA, Ressler KJ, Hammack SE, May V. Missig G, et al. Biol Psychiatry. 2017 Apr 15;81(8):671-682. doi: 10.1016/j.biopsych.2016.08.025. Epub 2016 Aug 29. Biol Psychiatry. 2017. PMID: 28057459 Free PMC article. - Pituitary Adenylate Cyclase-Activating Peptide in the Central Amygdala Causes Anorexia and Body Weight Loss via the Melanocortin and the TrkB Systems.
Iemolo A, Ferragud A, Cottone P, Sabino V. Iemolo A, et al. Neuropsychopharmacology. 2015 Jul;40(8):1846-55. doi: 10.1038/npp.2015.34. Epub 2015 Feb 4. Neuropsychopharmacology. 2015. PMID: 25649277 Free PMC article. - Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies.
Hammack SE, May V. Hammack SE, et al. Biol Psychiatry. 2015 Aug 1;78(3):167-77. doi: 10.1016/j.biopsych.2014.12.003. Epub 2014 Dec 9. Biol Psychiatry. 2015. PMID: 25636177 Free PMC article. Review. - PACAP orchestration of stress-related responses in neural circuits.
Boucher MN, May V, Braas KM, Hammack SE. Boucher MN, et al. Peptides. 2021 Aug;142:170554. doi: 10.1016/j.peptides.2021.170554. Epub 2021 Apr 15. Peptides. 2021. PMID: 33865930 Free PMC article. Review.
Cited by
- PACAP regulates neuroendocrine and behavioral stress responses via CRF-containing neurons of the rat hypothalamic paraventricular nucleus.
Ebner K, Fontebasso V, Ferro F, Singewald N, Hannibal J. Ebner K, et al. Neuropsychopharmacology. 2024 Oct 29. doi: 10.1038/s41386-024-02016-9. Online ahead of print. Neuropsychopharmacology. 2024. PMID: 39472527 - Flavonoids and Alzheimer's disease: reviewing the evidence for neuroprotective potential.
Al Amin M, Dehbia Z, Nafady MH, Zehravi M, Kumar KP, Haque MA, Baig MS, Farhana A, Khan SL, Afroz T, Koula D, Tutone M, Nainu F, Ahmad I, Emran TB. Al Amin M, et al. Mol Cell Biochem. 2024 Apr 3. doi: 10.1007/s11010-023-04922-w. Online ahead of print. Mol Cell Biochem. 2024. PMID: 38568359 Review. - The functional heterogeneity of PACAP: Stress, learning, and pathology.
Rajbhandari AK, Barson JR, Gilmartin MR, Hammack SE, Chen BK. Rajbhandari AK, et al. Neurobiol Learn Mem. 2023 Sep;203:107792. doi: 10.1016/j.nlm.2023.107792. Epub 2023 Jun 25. Neurobiol Learn Mem. 2023. PMID: 37369343 Free PMC article. Review. - Feeding crocin ameliorate cognitive dysfunction, oxidative stress and neuroinflammation induced by unpredictable chronic mild stress in rats.
Abbaszade-Cheragheali A, Kakhki S, Khatibi SR, Hosseini M, Navari F, Beheshti F. Abbaszade-Cheragheali A, et al. Inflammopharmacology. 2023 Aug;31(4):2079-2090. doi: 10.1007/s10787-023-01250-9. Epub 2023 Jun 1. Inflammopharmacology. 2023. PMID: 37261629 - The effects of self-selected background music and task difficulty on task engagement and performance in a visual vigilance task.
Homann LA, Drody AC, Smilek D. Homann LA, et al. Psychol Res. 2023 Nov;87(8):2460-2476. doi: 10.1007/s00426-023-01836-6. Epub 2023 May 27. Psychol Res. 2023. PMID: 37243700
References
- Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Frontiers in Neuroendocrinology. 1995;16(1):53–88. - PubMed
- Morley JE, Horowitz M, Morley PMK, Flood JF. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides. 1992;13(6):1133–1135. - PubMed
- Chance WT, Thompson H, Thomas I, Fischer JE. Anorectic and neurochemical effects of pituitary adenylate cyclase activating polypeptide in rats. Peptides. 1995;16(8):1511–1516. - PubMed
- Murase T, Kondo K, Otake K, Oiso Y. Pituitary adenylate cyclase-activating polypeptide stimulates arginine vasopressin release in conscious rats. Neuroendocrinology. 1993;57(6):1092–1096. - PubMed
- Nomura M, Ueta Y, Serino R, Yamamoto Y, Shibuya I, Yamashita H. Effects of centrally administered pituitary adenylate cyclase-activating polypeptide on c-fos gene expression and heteronuclear RNA for vasopressin in rat paraventricular and supraoptic nuclei. Neuroendocrinology. 1999;69(3):167–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases