Probing the flavivirus membrane fusion mechanism by using monoclonal antibodies - PubMed (original) (raw)
Probing the flavivirus membrane fusion mechanism by using monoclonal antibodies
Karin Stiasny et al. J Virol. 2007 Oct.
Abstract
In this study, we investigated in a flavivirus model (tick-borne encephalitis virus) the mechanisms of fusion inhibition by monoclonal antibodies directed to the different domains of the fusion protein (E) and to different sites within each of the domains by using in vitro fusion assays. Our data indicate that, depending on the location of their binding sites, the monoclonal antibodies impaired early or late stages of the fusion process, by blocking the initial interaction with the target membrane or by interfering with the proper formation of the postfusion structure of E, respectively. These data provide new insights into the mechanisms of flavivirus fusion inhibition by antibodies and their possible contribution to virus neutralization.
Figures
FIG. 1.
Schematic model of the flavivirus fusion process (A to E) and ribbon diagrams of the sE prefusion dimer (F and G) and postfusion trimer (H) of TBEV. E protein DI, red; E DII, yellow; E DIII, blue; FP, orange; stem, purple; transmembrane anchor, green; viral membrane, blue; target membrane, gray. (A) Metastable E dimer on the surface of native virions. (B) Low pH-induced dissociation of the dimer and insertion of the FP into the target membrane. (C) Relocation of DIII leading to hairpin formation, trimerization, and “zippering” of the stem along the body of the trimer. (D) Hemifusion intermediate. Only the contacting membrane leaflets are fused. (E) Fusion pore formation. In the final postfusion conformation, the FPs and the membrane anchors are juxtaposed in the fused membrane. (F) Side view and (G) top view of the sE dimer. (H) Side view of the sE trimer. The gray balls show the positions of mutations that affected binding of MAbs. The position of the carboxy terminus of sE is indicated by a purple arrow and labeled COO−.
FIG. 2.
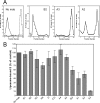
Low pH-induced fusion of pyrene-labeled TBEV in the absence (No mab) or presence of MAbs in a pyrene excimer fusion assay. Pyrene-labeled TBEV virions were preincubated with each of the MAbs overnight at 4°C, liposomes were added, the mixture was acidified, and the change in pyrene excimer fluorescence was monitored continuously for 60 s. The fusion curves were averaged, and the data shown are from at least three independent experiments. (A) Abolition of fusion (MAbs A3, A4, IC3). (B) No significant effect on fusion (MAbs B2, B3). (C) Reduction of the extent of fusion (MAbs A1, A2, A5). (D) Reduction of both rate and extent of fusion (MAbs IE3, i2, B1, B4).
FIG. 3.
Low pH-induced coflotation of TBEV with liposomes in the absence or presence of MAbs. TBEV virions were preincubated with each of the MAbs overnight at 4°C, liposomes were added, and the mixture was acidified, back-neutralized, and then subjected to centrifugation in sucrose step gradients. (A) Representative examples of coflotation experiments. From left, panel 1, coflotation without MAb (No mab) at pH 5.4 (solid line) and pH 7.4 (dotted line). Panels 2 to 4, coflotation in the presence of MAbs at pH 5.4. B2 (panel 2; no effect), A3 (panel 3; strong effect), and A2 (panel 4; intermediate effect). (B) Results of coflotation experiments with each of the MAbs, expressed as percentages of E protein bound to liposomes at pH 5.4 in comparison to the control without a MAb. The data are the averages from at least two independent experiments; the error bars represent the standard errors of the means.
FIG. 4.
(A) Blocking ELISA in the absence of detergent with native virions and MAbs A1, A2, A4, B2, and B4 as described previously (27). A predetermined fixed dilution of the respective MAb was incubated with decreasing concentrations of native TBEV. The mixture was then added to microtiter plates that had been coated with purified virus at a concentration of 0.5 μg/ml, a procedure that leads to the exposure of the FP loop, allowing its reaction with FP-specific MAbs (27). Antibody that was not blocked by the antigen in solution bound to the solid-phase antigen and was detected using a peroxidase-labeled rabbit anti-mouse immunoglobulin G (27). Results are expressed as percentages of the absorbance value obtained with each MAb in the absence of a blocking antigen. The data are representative of results of at least two independent experiments. (B) Four-layer ELISA with TBEV and MAb A1 to analyze the transient exposure of the A1 binding site upon acidification. Native TBEV in phosphate-buffered saline (pH 7.4; protein concentration, 0.5 μg/ml) was captured by polyclonal anti-TBEV immunoglobulin G for 1 h at 37°C as described previously (27). A1 epitope exposure was tested with the following combinations of pH and biotin-labeled MAb: column A, A1 was added in phosphate-buffered saline (pH 7.4); column B, A1 was added in MES buffer (pH 5.5; 50 mM MES, 100 mM NaCl); column C, the captured virus was exposed to MES buffer (pH 5.5) for 10 min followed by MAb A1 in the same buffer. After incubation for 1 h at 37°C, bound antibodies were detected by using streptavidin-peroxidase (Sigma-Aldrich). The data are the averages from five independent experiments performed in duplicate, and the error bars represent the standard errors of the means.
Similar articles
- Inhibiting Human Parainfluenza Virus Infection by Preactivating the Cell Entry Mechanism.
Bottom-Tanzer SF, Rybkina K, Bell JN, Alabi CA, Mathieu C, Lu M, Biswas S, Vasquez M, Porotto M, Melero JA, Más V, Moscona A. Bottom-Tanzer SF, et al. mBio. 2019 Feb 19;10(1):e02900-18. doi: 10.1128/mBio.02900-18. mBio. 2019. PMID: 30782664 Free PMC article. - Molecular Basis of a Protective/Neutralizing Monoclonal Antibody Targeting Envelope Proteins of both Tick-Borne Encephalitis Virus and Louping Ill Virus.
Yang X, Qi J, Peng R, Dai L, Gould EA, Gao GF, Tien P. Yang X, et al. J Virol. 2019 Apr 3;93(8):e02132-18. doi: 10.1128/JVI.02132-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30760569 Free PMC article. - Mechanism of neutralization of herpes simplex virus by antibodies directed at the fusion domain of glycoprotein B.
Cairns TM, Fontana J, Huang ZY, Whitbeck JC, Atanasiu D, Rao S, Shelly SS, Lou H, Ponce de Leon M, Steven AC, Eisenberg RJ, Cohen GH. Cairns TM, et al. J Virol. 2014 Mar;88(5):2677-89. doi: 10.1128/JVI.03200-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352457 Free PMC article. - The entry machinery of flaviviruses.
Heinz FX, Stiasny K, Allison SL. Heinz FX, et al. Arch Virol Suppl. 2004;(18):133-7. doi: 10.1007/978-3-7091-0572-6_11. Arch Virol Suppl. 2004. PMID: 15119768 Review. - Structures and mechanisms in flavivirus fusion.
Heinz FX, Allison SL. Heinz FX, et al. Adv Virus Res. 2000;55:231-69. doi: 10.1016/s0065-3527(00)55005-2. Adv Virus Res. 2000. PMID: 11050944 Free PMC article. Review.
Cited by
- Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus.
Gromowski GD, Barrett ND, Barrett AD. Gromowski GD, et al. J Virol. 2008 Sep;82(17):8828-37. doi: 10.1128/JVI.00606-08. Epub 2008 Jun 18. J Virol. 2008. PMID: 18562544 Free PMC article. - Novel approaches for the rapid development of rationally designed arbovirus vaccines.
van Bree JWM, Visser I, Duyvestyn JM, Aguilar-Bretones M, Marshall EM, van Hemert MJ, Pijlman GP, van Nierop GP, Kikkert M, Rockx BHG, Miesen P, Fros JJ. van Bree JWM, et al. One Health. 2023 May 13;16:100565. doi: 10.1016/j.onehlt.2023.100565. eCollection 2023 Jun. One Health. 2023. PMID: 37363258 Free PMC article. - Hantavirus Gn and Gc envelope glycoproteins: key structural units for virus cell entry and virus assembly.
Cifuentes-Muñoz N, Salazar-Quiroz N, Tischler ND. Cifuentes-Muñoz N, et al. Viruses. 2014 Apr 21;6(4):1801-22. doi: 10.3390/v6041801. Viruses. 2014. PMID: 24755564 Free PMC article. Review. - Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection.
Pierson TC, Diamond MS. Pierson TC, et al. Expert Rev Mol Med. 2008 May 12;10:e12. doi: 10.1017/S1462399408000665. Expert Rev Mol Med. 2008. PMID: 18471342 Free PMC article. Review. - An Absolutely Conserved Tryptophan in the Stem of the Envelope Protein E of Flaviviruses Is Essential for the Formation of Stable Particles.
Medits I, Heinz FX, Stiasny K. Medits I, et al. Viruses. 2021 Aug 30;13(9):1727. doi: 10.3390/v13091727. Viruses. 2021. PMID: 34578308 Free PMC article.
References
- Burke, D. S., and T. P. Monath. 2001. Flaviviruses, p. 1043-1126. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- Corver, J., A. Ortiz, S. L. Allison, J. Schalich, F. X. Heinz, and J. Wilschut. 2000. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Virology 269:37-46. - PubMed
- Crowe, J. E., Jr., R. O. Suara, S. Brock, N. Kallewaard, F. House, and J. H. Weitkamp. 2001. Genetic and structural determinants of virus neutralizing antibodies. Immunol. Res. 23:135-145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources