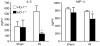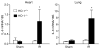Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia - PubMed (original) (raw)
Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia
M J Tracz et al. Kidney Int. 2007 Nov.
Abstract
Heme oxygenase-1 may exert cytoprotective effects. In this study we examined the sensitivity of heme oxygenase-1 knockout (HO-1(-/-)) mice to renal ischemia by assessing glomerular filtration rate (GFR) and cytokine expression in the kidney, and inflammatory responses in the systemic circulation and in vital extrarenal organs. Four hours after renal ischemia, the GFR of HO-1(-/-) mice was much lower than that of wild-type mice in the absence of changes in renal blood flow or cardiac output. Eight hours after renal ischemia, there was a marked induction of interleukin-6 (IL-6) mRNA and its downstream signaling effector, phosphorylated signal transducer and activator of transcription 3 (pSTAT3), in the kidney, lung, and heart in HO-1(-/-) mice. Systemic levels of IL-6 were markedly and uniquely increased in HO-1(-/-) mice after ischemia as compared to wild-type mice. The administration of an antibody to IL-6 protected against the renal dysfunction and mortality observed in HO-1(-/-) mice following ischemia. We suggest that the exaggerated production of IL-6, occurring regionally and systemically following localized renal ischemia, in an HO-1-deficient state may underlie the heightened sensitivity observed in this setting.
Figures
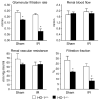
Figure 1. Renal hemodynamics in HO-1+/+ and HO-1−/− mice subjected to ischemia–reperfusion injury (IR) or sham ischemia (Sham)
The data demonstrate the following hemodynamic parameters: glomerular filtration rate (GFR), renal blood flow (RBF), renal vascular resistance (RVR), and filtration fraction (FF). n = 4 in each of the HO-1+/+ and HO-1−/− groups subjected to sham ischemia; n = 6 and n = 8 (GFR), and n = 5 and n = 7 (RBF, RVR, and FF) in HO-1+/+ and HO-1−/− groups subjected to IR respectively. *P< 0.05 versus HO-1+/+ mice subjected to the same condition.
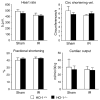
Figure 2. Cardiac function in HO-1+/+ and HO-1−/− mice subjected to ischemia–reperfusion injury (IR) or sham ischemia (Sham)
The data demonstrate the following: heart rate (beats per minute), circumferential shortening velocity (circumferences/s), fractional shortening (%), and cardiac output (ml/min/30 g). n = 5 in each of the HO-1+/+ Sham and HO-1+/+ IR groups, and n = 6 in each of HO-1−/− Sham and HO-1−/− IR groups.
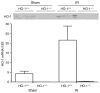
Figure 3. HO-1 expression in the kidney 4 h after sham or IR injury
Upper panel: Western analysis for HO-1 protein expression in HO-1+/+ and HO-1−/− mice subjected to ischemia–reperfusion injury (IR) or sham ischemia (Sham). Each lane represents protein extracted from a single kidney of an individual mouse. Equivalency of protein loading was verified by Ponceau staining (not shown). Lower panel: HO-1 mRNA by quantitative real-time reverse transcriptase-PCR in HO-1+/+ and HO-1−/− mice subjected to ischemia–reperfusion injury (IR) or sham ischemia (Sham). n = 4 in each of the HO-1+/+ Sham and HO-1−/− Sham groups, and n = 6 and n = 8 in HO-1+/+ IR and HO-1−/− IR groups respectively.
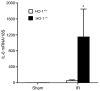
Figure 4. IL-6 mRNA determination by quantitative real-time reverse transcriptase-PCR in the kidney in HO-1+/+ and HO-1−/− mice subjected to ischemia–reperfusion injury (IR) or sham ischemia (Sham)
n = 4 and n = 5 in the HO-1+/+ Sham and HO-1−/− Sham groups respectively, and n = 8 and n = 7 in the HO-1+/+ IR and HO-1−/− IR groups respectively. *P< 0.05 versus HO-1+/+ mice subjected to the same condition.
Figure 5. Western analysis for pSTAT3 and STAT3 expression in the kidney in HO-1+/+ and HO-1−/− mice subjected to ischemia–reperfusion injury (IR) or sham ischemia (Sham)
Each lane represents protein extracted from a single kidney of an individual mouse, and equivalency of protein loading was verified by immunoblotting for _β_-actin.
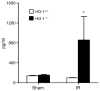
Figure 6. Serum levels of IL-6 in HO-1+/+ and HO-1−/− mice subjected to ischemia–reperfusion injury (IR) or sham ischemia (Sham)
n = 4 and n = 5 in the HO-1+/+ Sham and HO-1−/− Sham groups respectively, and n = 8 and n = 7 in the HO-1+/+ IR and HO-1−/− IR groups respectively. *P< 0.05 versus HO-1+/+ mice subjected to the same condition.
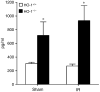
Figure 7. Serum levels of IL-12 (p40) in HO-1+/+ and HO-1−/− mice subjected to ischemia–reperfusion injury (IR) or sham ischemia (Sham)
n = 4 and n = 5 in the HO-1+/+ Sham and HO-1−/− Sham groups respectively, and n = 8 and n = 7 in the HO-1+/+ IR and HO-1−/− IR groups respectively. *P< 0.05 versus HO-1+/+ mice subjected to the same condition.
Figure 8. Serum levels of IL-5 and macrophage inflammatory protein (MIP)-1a in HO-1+/+ and HO-1−/− mice subjected to ischemia–reperfusion injury (IR) or sham ischemia (Sham)
n = 4 and n = 5 in the HO-1+/+ Sham and HO-1−/− Sham groups respectively, and n = 8 and n = 7 in the HO-1+/+ IR and HO-1−/− IR groups respectively. *P< 0.05 versus HO-1+/+ mice subjected to the same condition.
Figure 9. IL-6 mRNA determination by quantitative real-time reverse transcriptase-PCR in the heart and lung in HO-1+/+ and HO-1−/− mice subjected to ischemia–reperfusion injury (IR) or sham ischemia (Sham)
For studies in either organ, n = 4 and n = 5 in the HO-1+/+ Sham and HO-1−/− Sham groups respectively, and n = 8 and n = 7 in the HO-1+/+ IR and HO-1−/− IR groups respectively. *P< 0.05 versus HO-1+/+ mice subjected to the same condition.
Figure 10. Western analysis for pSTAT3 and STAT3 expression in the lung in HO-1+/+ and HO-1−/− mice subjected to ischemia–reperfusion injury (IR) or sham ischemia (Sham)
Each lane represents protein extracted from a single lung of an individual mouse, and equivalency of protein loading was verified by immunoblotting for _β_-actin.
Similar articles
- Hepatic ischemia-reperfusion induces renal heme oxygenase-1 via NF-E2-related factor 2 in rats and mice.
Tanaka Y, Maher JM, Chen C, Klaassen CD. Tanaka Y, et al. Mol Pharmacol. 2007 Mar;71(3):817-25. doi: 10.1124/mol.106.029033. Epub 2006 Dec 6. Mol Pharmacol. 2007. PMID: 17151289 - Critical involvement of Th1-related cytokines in renal injuries induced by ischemia and reperfusion.
de Paiva VN, Monteiro RM, Marques Vde P, Cenedeze MA, Teixeira Vde P, dos Reis MA, Pacheco-Silva A, Câmara NO. de Paiva VN, et al. Int Immunopharmacol. 2009 Jun;9(6):668-72. doi: 10.1016/j.intimp.2008.11.012. Epub 2008 Dec 16. Int Immunopharmacol. 2009. PMID: 19095086 - Heme oxygenase-2 protects against ischemic acute kidney injury: influence of age and sex.
Nath KA, Garovic VD, Grande JP, Croatt AJ, Ackerman AW, Farrugia G, Katusic ZS, Belcher JD, Vercellotti GM. Nath KA, et al. Am J Physiol Renal Physiol. 2019 Sep 1;317(3):F695-F704. doi: 10.1152/ajprenal.00085.2019. Epub 2019 Jun 19. Am J Physiol Renal Physiol. 2019. PMID: 31215802 Free PMC article. - Heme Oxygenase-1 Regulates Myeloid Cell Trafficking in AKI.
Hull TD, Kamal AI, Boddu R, Bolisetty S, Guo L, Tisher CC, Rangarajan S, Chen B, Curtis LM, George JF, Agarwal A. Hull TD, et al. J Am Soc Nephrol. 2015 Sep;26(9):2139-51. doi: 10.1681/ASN.2014080770. Epub 2015 Feb 12. J Am Soc Nephrol. 2015. PMID: 25677389 Free PMC article. - Viral interleukin-10 gene transfer prevents liver ischemia-reperfusion injury: Toll-like receptor-4 and heme oxygenase-1 signaling in innate and adaptive immunity.
Ke B, Shen XD, Tsuchihashi S, Gao F, Araujo JA, Busuttil RW, Ritter T, Kupiec-Weglinski JW. Ke B, et al. Hum Gene Ther. 2007 Apr;18(4):355-66. doi: 10.1089/hum.2007.181. Hum Gene Ther. 2007. PMID: 17439357
Cited by
- Acute Kidney Injury as a Condition of Renal Senescence.
Andrade L, Rodrigues CE, Gomes SA, Noronha IL. Andrade L, et al. Cell Transplant. 2018 May;27(5):739-753. doi: 10.1177/0963689717743512. Epub 2018 Apr 27. Cell Transplant. 2018. PMID: 29701108 Free PMC article. Review. - Proximal tubule-targeted heme oxygenase-1 in cisplatin-induced acute kidney injury.
Bolisetty S, Traylor A, Joseph R, Zarjou A, Agarwal A. Bolisetty S, et al. Am J Physiol Renal Physiol. 2016 Mar 1;310(5):F385-94. doi: 10.1152/ajprenal.00335.2015. Epub 2015 Dec 16. Am J Physiol Renal Physiol. 2016. PMID: 26672618 Free PMC article. - Progressive histone alterations and proinflammatory gene activation: consequences of heme protein/iron-mediated proximal tubule injury.
Zager RA, Johnson AC. Zager RA, et al. Am J Physiol Renal Physiol. 2010 Mar;298(3):F827-37. doi: 10.1152/ajprenal.00683.2009. Epub 2009 Dec 23. Am J Physiol Renal Physiol. 2010. PMID: 20032114 Free PMC article. - Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice: effects on macrophage viability and tissue iron distribution.
Kovtunovych G, Eckhaus MA, Ghosh MC, Ollivierre-Wilson H, Rouault TA. Kovtunovych G, et al. Blood. 2010 Dec 23;116(26):6054-62. doi: 10.1182/blood-2010-03-272138. Epub 2010 Sep 15. Blood. 2010. PMID: 20844238 Free PMC article. - Distant Organ Dysfunction in Acute Kidney Injury: A Review.
Lee SA, Cozzi M, Bush EL, Rabb H. Lee SA, et al. Am J Kidney Dis. 2018 Dec;72(6):846-856. doi: 10.1053/j.ajkd.2018.03.028. Epub 2018 Jun 14. Am J Kidney Dis. 2018. PMID: 29866457 Free PMC article. Review.
References
- Rabb H, O’Meara YM, Maderna P, et al. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51:1463–1468. - PubMed
- Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. - PubMed
- Kielar ML, Rohan Jeyarajah D, Lu CY. The regulation of ischemic acute renal failure by extrarenal organs. Curr Opin Nephrol Hypertens. 2002;11:451–457. - PubMed
- Burne-Taney MJ, Rabb H. The role of adhesion molecules and T cells in ischemic renal injury. Curr Opin Nephrol Hypertens. 2003;12:85–90. - PubMed
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK47060/DK/NIDDK NIH HHS/United States
- R01 DK047060/DK/NIDDK NIH HHS/United States
- R01 DK068545/DK/NIDDK NIH HHS/United States
- R01 DK070124/DK/NIDDK NIH HHS/United States
- HL64822/HL/NHLBI NIH HHS/United States
- R01 HL064822/HL/NHLBI NIH HHS/United States
- DK68545/DK/NIDDK NIH HHS/United States
- R01 DK070124-04/DK/NIDDK NIH HHS/United States
- R37 DK047060/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous