Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin - PubMed (original) (raw)
Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin
Yin Xia et al. Blood. 2008.
Abstract
Hemojuvelin (HJV) is a coreceptor for bone morphogenetic protein (BMP) signaling that regulates hepcidin expression and iron metabolism. However, the precise combinations of BMP ligands and receptors used by HJV remain unknown. HJV has also been demonstrated to bind to neogenin, but it is not known whether this interaction has a role in regulating hepcidin expression. In the present study, we show that BMP-2, BMP-4, and BMP-6 are endogenous ligands for HJV in hepatoma-derived cell lines, and that all 3 of these ligands are expressed in human liver. We demonstrate in vitro that HJV selectively uses the BMP type II receptors ActRIIA and BMPRII, but not ActRIIB, and HJV enhances utilization of ActRIIA by BMP-2 and BMP-4. Interestingly, ActRIIA is the predominant BMP type II receptor expressed in human liver. While HJV can use all 3 BMP type I receptors (ALK2, ALK3, and ALK6) in vitro, only ALK2 and ALK3 are detected in human liver. Finally, we show that HJV-induced BMP signaling and hepcidin expression are not altered by neogenin overexpression or by inhibition of endogenous neogenin expression. Thus, HJV-mediated BMP signaling and hepcidin regulation occur via a distinct subset of BMP ligands and BMP receptors, independently of neogenin.
Figures
Figure 1
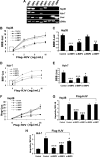
Impact of siRNA-mediated specific inhibition of BMP ligand expression on BMP signaling and hepcidin expression induced by HJV in Hep3B and Huh-7 cells. (A) Total RNA from Hep3B and Huh-7 cells and human liver was extracted for RT-PCR to determine the expression of BMP-2, BMP-4, BMP-5, BMP-6, BMP-7, GDF-5, GDF-6, and BMP-9. Purified plasmid cDNAs containing these ligands were used as positive controls. (B-E) Hep3B cells (B,C) or Huh-7 cells (D,E) were transfected with the BMP-responsive firefly luciferase reporter (BRE-Luc) and pRL-TK Renilla luciferase vector, either alone or with increasing amounts of Flag-HJV cDNA, in combination with control siRNA or siRNA specific for BMP-2, BMP-4, BMP-6, or BMP-7 (Control, si-BMP-2, siBMP-4, siBMP-6, and si-BMP-7; 60 nM) for 46 hours prior to measurement of luciferase activity. Firefly luciferase values were normalized for transfection efficiency relative to Renilla activity. Values shown are the means of triplicate measurements plus or minus SD. Basal BRE luciferase activities in the absence or presence of BMP-2, BMP-4, BMP-6, and BMP-7 siRNAs (dotted square in panels B and D) were replotted in panels C and E. (F) Hep3B cells were transfected with the hepcidin promoter firefly luciferase reporter (Hep-Luc) and pRL-TK Renilla luciferase vector, either alone or with increasing amounts of Flag-HJV cDNA, in combination with control siRNA or si-BMP-2, si-BMP-4, si-BMP-6, or si-BMP-7 at 60 nM. After 46 hours, cell lysates were analyzed for luciferase activity as in panels B-E. (G,H) Hep3B cells (G) or Huh-7 cells (H) were transfected with empty vector (pcDNA3) and control siRNA or with Flag-HJV cDNA in combination with control siRNA, si-BMP-2, si-BMP-4, si-BMP-6, or si-BMP-7 at 60 nM. After 46 hours, cells were collected for RNA extraction and real time RT-PCR analyses to quantify hepcidin and RPL19 mRNA levels. Hepcidin expression values were normalized to RPL19 mRNA levels. Values shown are the means of triplicate measurements plus or minus SD. *P < .05; **P < .01; ***P < .001.
Figure 2
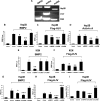
Impact of siRNA-mediated specific inhibition of BMP type II receptor expression on BMP signaling and hepcidin expression induced by HJV in Hep3B and KGN cells. (A) Expression of BMP type II receptors BMPRII, ActRIIA, and ActRIIB in Hep3B cells and human liver (RT-PCR). KGN cells were used as a positive control. (B-F) Hep3B (B-D) or KGN cells (E,F) were transfected with BRE-Luc (B,C,E,F) or the activin-responsive firefly luciferase reporter (CAGA-Luc; panel D) and pRL-TK Renilla luciferase vector in combination with control siRNA or siRNA specific for BMPRII, ActRIIA, or ActRIIB (60 nM). Cells were incubated in the absence or presence of 20 ng/mL BMP-2 (B,E) or activin A (D) or cotransfected with HJV cDNA (8 ng/mL; panels C,F). Cell lysates were analyzed for luciferase activity as in Figure 1B-E. (G,H) Hep3B cells were transfected with the hepcidin promoter reporter construct (Hep-Luc) and pRL-TK, in combination with control siRNA or siRNA specific for BMPRII, ActRIIA, or ActRIIB (40 nM). Cells were incubated in the absence or presence of 20 ng/mL BMP-2 (G), or after cotransfection with HJV cDNA (8 ng/mL; panel H) prior to measurement of luciferase activity. (I) Hep3B cells were transfected with empty vector (pcDNA3) and control siRNA, or with Flag-HJV cDNA in combination with control siRNA, si-BMPRII, si-ActRIIA, or si-ActRIIB at 40 nM. At 46 hours after transfection, hepcidin relative to RPL19 mRNA levels were quantified by real-time RT-PCR as in Figure 1G,H. Values shown are the means of triplicate measurements plus or minus SD. *P < .05; **P < .01; ***P < .001.
Figure 3
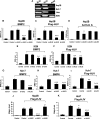
Impact of siRNA-mediated specific inhibition of BMP type I receptor expression on BMP signaling and hepcidin expression induced by HJV in Hep3B, KGN, and Huh-7 cells. (A) Expression of BMP type I receptors ALK2, ALK3, and ALK6 in Hep3B, Huh-7, and KGN cells, and human liver (RT-PCR). (B-I) Hep3B (B-D), KGN (E,F), or Huh-7 cells (G-I) were transfected with BRE-Luc or CAGA-Luc and pRL-TK Renilla luciferase vector, in combination with control siRNA or siRNA specific for ALK2, ALK3, and ALK6 (60 nM). Cells were incubated in the absence or presence of 20 ng/mL BMP-2 (B,E,G), 20 ng/mL activin A (D), or 20 ng/mL BMP-6 (H), or after cotransfection with 8 ng/mL HJV cDNA (C,F,I). Cell lysates were analyzed for luciferase activity as in Figure 1B-E. (J,K) Hep3B (J) or Huh-7 cells (K) were transfected with Hep-Luc and pRL-TK, in combination with control siRNA or siRNA specific for ALK2, ALK3, or ALK6 (60 nM), in the absence or presence of HJV cDNA (8 ng/mL) for 46 hours prior to measurement of luciferase activity. Values shown are the means of triplicate measurements plus or minus SD. *P < .05; **P < .01; ***P < .001.
Figure 4
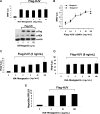
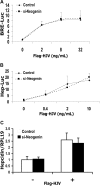
HJV-mediated BMP signaling and hepcidin expression are not altered by neogenin overexpression in Hep3B cells. (A) Hep3B cells were transfected with BRE-Luc and pRL-TK, either alone or with a fixed amount of Flag-HJV cDNA in combination with increasing amounts of HA-neogenin cDNA for 46 hours. Cell lysates were analyzed for luciferase activity as in Figure 1B-E (top panel) or by Western blot in succession with Flag antibody (M5; α-Flag), neogenin antibody (α-NEN), and actin antibody (α-actin, as a loading control; bottom panel). (B) Hep3B cells were transfected with BRE-Luc and pRL-TK, either alone or with fixed amount of HA-neogenin cDNA (40 ng/mL), in combination with increasing amounts of Flag-HJV cDNA. Cell lysates were analyzed for luciferase activity as in Figure 1B-E. (C,D) Hep3B cells were transfected with Hep-Luc and pRL-TK, either alone or with Flag-HJV at 2 ng/mL (C) or 8 ng/mL (D), in combination with increasing amounts of HA-neogenin cDNA. Cell lysates were analyzed for luciferase activity as in Figure 1B-E. (E) Hep3B cells were transfected with empty vector (pcDNA3) or Flag-HJV cDNA in combination with increasing amount of HA-neogenin cDNA. After 46 hours, hepcidin relative to RPL19 mRNA levels were quantified by real-time RT-PCR as in Figure 1G,H. Values shown are the means of triplicate measurements plus or minus SD.
Figure 5
HJV-mediated BMP signaling and hepcidin expression are not altered by inhibition of endogenous neogenin expression in Hep3B cells. (A,B) Hep3B cells were transfected with BRE-Luc (A) or Hep-Luc (B) and pRL-TK Renilla luciferase vector, either alone or with increasing amounts of Flag-HJV cDNA, in combination with control siRNA or neogenin siRNA. After 46 hours, cell lysates were analyzed for luciferase activity as in Figure 1B-F. (C) Hep3B cells were transfected with empty vector (pcDNA3) or Flag-HJV cDNA in combination with control siRNA or si-neogenin. After 46 hours, hepcidin relative to RPL19 mRNA levels were quantified by real-time RT-PCR as in Figure 1G,H. Values shown are the means of triplicate measurements plus or minus SD.
Figure 6
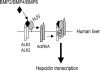
Schematic diagram depicting HJV action in regulating hepcidin expression in human liver. ActRIIA is the predominant BMP type II receptor, and ALK3 and ALK2 are predominant BMP type I receptors expressed in the human liver. HJV facilitates endogenous ligands BMP-2, BMP-4, and BMP-6 to signal through ActRIIA in combination with ALK3 and/or ALK2 to regulate hepcidin expression
Similar articles
- Neogenin Facilitates the Induction of Hepcidin Expression by Hemojuvelin in the Liver.
Zhao N, Maxson JE, Zhang RH, Wahedi M, Enns CA, Zhang AS. Zhao N, et al. J Biol Chem. 2016 Jun 3;291(23):12322-35. doi: 10.1074/jbc.M116.721191. Epub 2016 Apr 12. J Biol Chem. 2016. PMID: 27072365 Free PMC article. - Evidence that inhibition of hemojuvelin shedding in response to iron is mediated through neogenin.
Zhang AS, Anderson SA, Meyers KR, Hernandez C, Eisenstein RS, Enns CA. Zhang AS, et al. J Biol Chem. 2007 Apr 27;282(17):12547-56. doi: 10.1074/jbc.M608788200. Epub 2007 Mar 1. J Biol Chem. 2007. PMID: 17331953 - Neogenin inhibits HJV secretion and regulates BMP-induced hepcidin expression and iron homeostasis.
Lee DH, Zhou LJ, Zhou Z, Xie JX, Jung JU, Liu Y, Xi CX, Mei L, Xiong WC. Lee DH, et al. Blood. 2010 Apr 15;115(15):3136-45. doi: 10.1182/blood-2009-11-251199. Epub 2010 Jan 11. Blood. 2010. PMID: 20065295 Free PMC article. - Hemojuvelin: the hepcidin story continues.
Malyszko J. Malyszko J. Kidney Blood Press Res. 2009;32(2):71-6. doi: 10.1159/000208988. Epub 2009 Mar 14. Kidney Blood Press Res. 2009. PMID: 19287179 Review. - Bone morphogenic proteins in iron homeostasis.
Xiao X, Alfaro-Magallanes VM, Babitt JL. Xiao X, et al. Bone. 2020 Sep;138:115495. doi: 10.1016/j.bone.2020.115495. Epub 2020 Jun 23. Bone. 2020. PMID: 32585319 Free PMC article. Review.
Cited by
- Out of balance--systemic iron homeostasis in iron-related disorders.
Steinbicker AU, Muckenthaler MU. Steinbicker AU, et al. Nutrients. 2013 Aug 2;5(8):3034-61. doi: 10.3390/nu5083034. Nutrients. 2013. PMID: 23917168 Free PMC article. Review. - Targeting the hepcidin-ferroportin axis in the diagnosis and treatment of anemias.
Nemeth E. Nemeth E. Adv Hematol. 2010;2010:750643. doi: 10.1155/2010/750643. Epub 2009 Dec 24. Adv Hematol. 2010. PMID: 20066043 Free PMC article. - Angiotensin II alters the expression of duodenal iron transporters, hepatic hepcidin, and body iron distribution in mice.
Tajima S, Ikeda Y, Enomoto H, Imao M, Horinouchi Y, Izawa-Ishizawa Y, Kihira Y, Miyamoto L, Ishizawa K, Tsuchiya K, Tamaki T. Tajima S, et al. Eur J Nutr. 2015 Aug;54(5):709-19. doi: 10.1007/s00394-014-0749-1. Epub 2014 Aug 7. Eur J Nutr. 2015. PMID: 25096756 - Hepcidin Downregulation Correlates With Disease Aggressiveness And Immune Infiltration in Liver Cancers.
Wang J, Liu W, Li JC, Li M, Li B, Zhu R. Wang J, et al. Front Oncol. 2021 Jun 30;11:714756. doi: 10.3389/fonc.2021.714756. eCollection 2021. Front Oncol. 2021. PMID: 34277457 Free PMC article. - Potential roles of BMP9 in liver fibrosis.
Bi J, Ge S. Bi J, et al. Int J Mol Sci. 2014 Nov 11;15(11):20656-67. doi: 10.3390/ijms151120656. Int J Mol Sci. 2014. PMID: 25393508 Free PMC article. Review.
References
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. - PubMed
- Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. - PubMed
- Krause A, Neitz S, Magert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150. - PubMed
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. - PubMed
- Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 DK075846/DK/NIDDK NIH HHS/United States
- R01 DK-069533/DK/NIDDK NIH HHS/United States
- R01 DK069533/DK/NIDDK NIH HHS/United States
- R01 DK-071837/DK/NIDDK NIH HHS/United States
- K08 DK075846-02/DK/NIDDK NIH HHS/United States
- K08 DK-075846/DK/NIDDK NIH HHS/United States
- R01 DK071837/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases