FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis - PubMed (original) (raw)
FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis
Damian Medici et al. J Cell Biol. 2008.
Abstract
Fibroblast growth factor 23 (FGF-23) and Klotho are secretory proteins that regulate mineral-ion metabolism. Fgf-23(-/-) or Klotho(-/-) knockout mice exhibit several pathophysiological processes consistent with premature aging including severe atrophy of tissues. We show that the signal transduction pathways initiated by FGF-23-Klotho prevent tissue atrophy by stimulating proliferation and preventing apoptosis caused by excessive systemic vitamin D. Because serum levels of active vitamin D are greatly increased upon genetic ablation of Fgf-23 or Klotho, we find that these molecules have a dual role in suppression of apoptotic actions of vitamin D through both negative regulation of 1alpha-hydroxylase expression and phosphoinositide-3 kinase-dependent inhibition of caspase activity. These data provide new insights into the physiological roles of FGF-23 and Klotho.
Figures
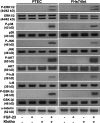
Figure 1.
Signaling events induced by FGF-23–Klotho. Immunoblotting showing that FGF-23 or Klotho alone have no effect on kinase activity in PTEC or FHs74Int cells. Combined effects of FGF-23 and Klotho show increased phosphorylation of ERK1/2, p38, JNK, AKT, IκB, and GSK-3β. α-Tubulin was used as a loading control.
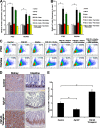
Figure 2.
FGF-23–Klotho signaling promotes cell proliferation. (A and B) ELISA analysis showing increased expression of the cell cycle proteins Cyclin D1 (A) and c-myc (B) upon exposure of cells to FGF-23 and Klotho. FGF-23 or Klotho alone had little effect on expression of these proteins. Addition of a small molecule Ras inhibitor nearly abolished up-regulation of these proteins by FGF-23–Klotho, whereas PI3K inhibitor had minimal effect. Graphs represent mean ± SD (n = 3). *, P < 0.001. (C) Flow cytometry analysis for BrdU incorporation under these conditions showing that cell proliferation correlated with the observed expression patterns for Cyclin D1 and c-myc. (D and E) Ki67 immunostaining showing that expression was lower in Fgf-23−/− mice and higher in FGF-23 transgenic mice. Intestinal tissue showed intense nuclear Ki67 staining (E), whereas the kidney tissue only stained in the cytoplasm. Bar, 100 μm. Graphs represent mean ± SD (n = 3). *, P < 0.05.
Figure 3.
FGF-23–Klotho prevents vitamin D–induced apoptosis. (A) ELISA analysis of 1α-hydroxylase expression showing no significant changes in PTEC cells exposed to FGF-23 or Klotho alone but greatly decreased levels when exposed to both FGF-23 and Klotho. Small molecule inhibitors against Ras and PI3K were sufficient to provide marginal rescue of this decrease in expression. No significant changes were found for treatment of FHs74Int cells. Graphs represent mean ± SD (n = 3). *, P < 0.05. (B) Flow cytometry analysis for TUNEL staining of cells exposed to exogenous vitamin D showing that it caused extremely high levels of apoptosis. Addition of FGF-23 and Klotho was sufficient to rescue most of the vitamin D–induced apoptosis, whereas FGF-23 or Klotho alone did not. PI3K inhibitor prevented this rescue, whereas Ras inhibitor had no effect. (C) ELISA for active caspase-3 levels, showing the same patterns as observed with the TUNEL analysis. Graphs represent mean ± SD (n = 3). *, P < 0.001.
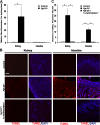
Figure 4.
Genetic ablation of 1α-hydroxylase prevents apoptosis and atrophy in tissues of Fgf-23−/− mice. (A) Real-time quantitative PCR analysis showing that gene expression levels of 1α-hydroxylase from kidney tissues of Fgf-23−/− mice is much higher than those of wild-type (control) mice. Graphs represent mean ± SD (kidney, n = 6; intestine, n = 5 ). *, P < 0.01. (B and C) TUNEL staining of tissue sections from kidney and intestine of Fgf-23−/− mice showing far higher levels of apoptosis than those of wild-type mice. Fgf-23−/−/1α-hydroxylase−/− double knockout mice show a complete rescue of the apoptosis seen in tissues of Fgf-23−/− mice. Bar, 100 μm. Graphs represent mean ± SD (n = 3). *, P < 0.001.
Similar articles
- Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process.
Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Razzaque MS, et al. FASEB J. 2006 Apr;20(6):720-2. doi: 10.1096/fj.05-5432fje. Epub 2006 Jan 25. FASEB J. 2006. PMID: 16436465 Free PMC article. - Premature aging in klotho mutant mice: cause or consequence?
Lanske B, Razzaque MS. Lanske B, et al. Ageing Res Rev. 2007 May;6(1):73-9. doi: 10.1016/j.arr.2007.02.002. Epub 2007 Feb 20. Ageing Res Rev. 2007. PMID: 17353153 Free PMC article. Review. - Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease.
de Borst MH, Vervloet MG, ter Wee PM, Navis G. de Borst MH, et al. J Am Soc Nephrol. 2011 Sep;22(9):1603-9. doi: 10.1681/ASN.2010121251. Epub 2011 Aug 18. J Am Soc Nephrol. 2011. PMID: 21852584 Free PMC article. - Regulation of fibroblast growth factor-23 signaling by klotho.
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Kurosu H, et al. J Biol Chem. 2006 Mar 10;281(10):6120-3. doi: 10.1074/jbc.C500457200. Epub 2006 Jan 25. J Biol Chem. 2006. PMID: 16436388 Free PMC article. - Klotho and aging.
Kuro-o M. Kuro-o M. Biochim Biophys Acta. 2009 Oct;1790(10):1049-58. doi: 10.1016/j.bbagen.2009.02.005. Epub 2009 Feb 20. Biochim Biophys Acta. 2009. PMID: 19230844 Free PMC article. Review.
Cited by
- Differential Regulatory Role of Soluble Klothos on Cardiac Fibrogenesis in Hypertension.
Liu X, Chen Y, McCoy CW, Zhao T, Quarles DL, Pi M, Bhattacharya SK, King G, Sun Y. Liu X, et al. Am J Hypertens. 2016 Oct;29(10):1140-7. doi: 10.1093/ajh/hpw062. Epub 2016 Aug 19. Am J Hypertens. 2016. PMID: 27543985 Free PMC article. - Investigation on urinary and serum alpha klotho in dogs with chronic kidney disease.
Yi HJ, Lee JB, Lee KP, Oh YI, Song KH, Seo KW. Yi HJ, et al. BMC Vet Res. 2020 Jul 16;16(1):246. doi: 10.1186/s12917-020-02458-5. BMC Vet Res. 2020. PMID: 32677951 Free PMC article. - Association between serum Klotho and the prevalence of osteoarthritis: A cross-sectional study from NHANES 2007-2016.
Qiu Y, Yin H, Meng J, Cai Y, Huang J, Zheng X, Yao J, Li J. Qiu Y, et al. PLoS One. 2024 Nov 18;19(11):e0312562. doi: 10.1371/journal.pone.0312562. eCollection 2024. PLoS One. 2024. PMID: 39556550 Free PMC article. - Phosphate toxicity: new insights into an old problem.
Razzaque MS. Razzaque MS. Clin Sci (Lond). 2011 Feb;120(3):91-7. doi: 10.1042/CS20100377. Clin Sci (Lond). 2011. PMID: 20958267 Free PMC article. Review. - FGF-23 protects cell function and viability in murine pancreatic islets challenged by glucolipotoxicity.
Pajaziti B, Yosy K, Steinberg OV, Düfer M. Pajaziti B, et al. Pflugers Arch. 2023 Mar;475(3):309-322. doi: 10.1007/s00424-022-02772-x. Epub 2022 Nov 28. Pflugers Arch. 2023. PMID: 36437429 Free PMC article.
References
- Bennasroune, A., A. Gardin, D. Aunis, G. Cremel, and P. Hubert. 2004. Tyrosine kinase receptors as attractive targets of cancer therapy. Crit. Rev. Oncol. Hematol. 50:23–28. - PubMed
- Christakos, S., P. Dhawan, Q. Shen, X. Peng, B. Benn, and Y. Zhong. 2006. New insights into the mechanisms involved in the pleiotropic action of 1,25dihydroxyvitamin D3. Ann. N. Y. Acad. Sci. 1068:194–203. - PubMed
- Dardenne, O., J. Prudhomme, A. Arabian, F.H. Glorieux, and R. St-Arunaud. 2001. Targeted inactivation of the 25-hydroxyvitamin D3-1α-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 142:3135–3141. - PubMed
- Deeb, K.K., D.L. Trump, and C.S. Johnson. 2007. Vitamin D signaling pathways in cancer: potential for anticancer therapeutics. Nat. Rev. Cancer. 7:684–700. - PubMed
- Demay, M.B. 2006. Mechanism of vitamin D receptor action. Ann. N. Y. Acad. Sci. 1068:204–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-073944/PHS HHS/United States
- P01 AR048564/AR/NIAMS NIH HHS/United States
- R01 DK073944/DK/NIDDK NIH HHS/United States
- P01-AR048564/AR/NIAMS NIH HHS/United States
- R01-077276/PHS HHS/United States
- R01 DK077276/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases