Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation - PubMed (original) (raw)
. 2008 Nov-Dec;14(11-12):665-74.
doi: 10.2119/2008-00102.Catera. Epub 2008 Sep 25.
Gregg J Silverman, Katerina Hatzi, Till Seiler, Sebastien Didier, Lu Zhang, Maxime Hervé, Eric Meffre, David G Oscier, Helen Vlassara, R Hal Scofield, Yifang Chen, Steven L Allen, Jonathan Kolitz, Kanti R Rai, Charles C Chu, Nicholas Chiorazzi
Affiliations
- PMID: 19009014
- PMCID: PMC2582860
- DOI: 10.2119/2008-00102.Catera
Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation
Rosa Catera et al. Mol Med. 2008 Nov-Dec.
Abstract
Chronic lymphocytic leukemia (CLL) represents the outgrowth of a CD5(+) B cell. Its etiology is unknown. The structure of membrane Ig on CLL cells of unrelated patients can be remarkably similar. Therefore, antigen binding and stimulation could contribute to clonal selection and expansion as well as disease promotion. Initial studies suggest that CLL mAbs bind autoantigens. Since apoptosis can make autoantigens accessible for recognition by antibodies, and also create neo-epitopes by chemical modifications occurring naturally during this process, we sought to determine if CLL mAbs recognize autoantigens associated with apoptosis. In general, ~60% of CLL mAbs bound the surfaces of apoptotic cells, were polyreactive, and expressed unmutated IGHV. mAbs recognized two types of antigens: native molecules located within healthy cells, which relocated to the external cell surface during apoptosis; and/or neoantigens, generated by oxidation during the apoptotic process. Some of the latter epitopes are similar to those on bacteria and other microbes. Although most of the reactive mAbs were not mutated, the use of unmutated IGHV did not bestow autoreactivity automatically, since several such mAbs were not reactive. Particular IGHV and IGHV/D/J rearrangements contributed to autoantigen binding, although the presence and degree of reactivity varied based on specific structural elements. Thus, clonal expansion in CLL may be stimulated by autoantigens occurring naturally during apoptosis. These data suggest that CLL may derive from normal B cells whose function is to remove cellular debris, and also to provide a first line of defense against pathogens.
Figures
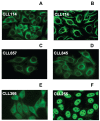
Figure 1
Immunoreactivity patterns of CLL mAbs with intracellular structures of live human cells. Healthy HEp-2 cells were fixed, permeabilized, and exposed to 50 μg/mL of mAbs 114 (A,B), 657 (C), 845 (D), 366 (E), and 255 (F). Binding was detected with FITC-labeled goat anti-human IgG. Standard immunofluorescence microscopy images (A,C–F). Confocal microscopy showing mAb 114 binding to fiberlike structures suggestive of cytoskeletal components (B). Studies by Chu et al. indicate that vimentin is the target of this mAb (49).
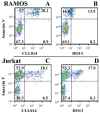
Figure 2
CLL mAbs bind apoptotic B and T cells. γ-irradiated RAMOS (upper) and Jurkat (lower) B and T cells were exposed under non-permeabilizing conditions to Annexin V-PE and mAbs 014 (A,C) or DO13 (B,D) (50 μg/mL) plus FITC-labeled goat anti-human IgG.
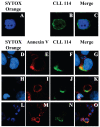
Figure 3
Differential binding of CLL mAbs to healthy versus apoptotic cells. (A–C) Viable Jurkat T cells were fixed, permeabilized, and exposed to SYTOX Orange and mAb 114 (50 μg/mL) plus FITC-labeled (Fab’)2 goat anti-human IgG. (D–G) Reactivity of mAb 114 with surfaces of camptothecin-treated Jurkat T cells was analyzed using cells that were not permeabilized and co-stained with SYTOX Orange and Annexin V-Alexa Fluor 647; reactivity with membranes of apoptotic blebs without (H–K) and with (L-O) DNA was determined similarly. Blue: nucleic acid binding by SYTOX Orange; red: membrane Annexin V binding; green: CLL mAb binding; yellow: co-localization of Annexin V and mAb binding.
Figure 4
Hierarchical cluster analysis of autoantigen microarray reactivity of CLL mAbs. Results represent a two-dimensional cluster analysis in which CLL mAbs with similar binding patterns were assigned by computational algorithm to adjacent positions. In the second dimension, a similar computational clustering of antigenic reactivities is shown. Heat map: bright red represents highest relative antibody activity level; bright blue the absence of relative activity level. Results denote reactivity with antigens bound by ≥ 3 mAbs.
Figure 5
CLL mAbs bind neoantigens created by oxidation. Reactivity of CLL mAbs (25 μg/mL) with oxidation-induced molecular adducts (MDA and HNE) of BSA was tested by ELISA.
Figure 6
Inhibition of CLL mAb binding to apoptotic cells. γ-Irradiated Jurkat T cells were exposed to CLL mAbs 114 or DO13 (25 μg/mL) plus FITC-labeled (Fab’)2 goat anti-IgG.(A–C and G–I) mAb binding without (red) or with (green) MDA-BSA (100 μg/mL). (D–F and J–L) mAb binding without (red) or with (green) native BSA (100 μg/mL).
Similar articles
- Characterization of structurally defined epitopes recognized by monoclonal antibodies produced by chronic lymphocytic leukemia B cells.
Seiler T, Woelfle M, Yancopoulos S, Catera R, Li W, Hatzi K, Moreno C, Torres M, Paul S, Dohner H, Stilgenbauer S, Kaufman MS, Kolitz JE, Allen SL, Rai KR, Chu CC, Chiorazzi N. Seiler T, et al. Blood. 2009 Oct 22;114(17):3615-24. doi: 10.1182/blood-2009-01-197822. Epub 2009 Aug 18. Blood. 2009. PMID: 19690339 Free PMC article. - Many chronic lymphocytic leukemia antibodies recognize apoptotic cells with exposed nonmuscle myosin heavy chain IIA: implications for patient outcome and cell of origin.
Chu CC, Catera R, Zhang L, Didier S, Agagnina BM, Damle RN, Kaufman MS, Kolitz JE, Allen SL, Rai KR, Chiorazzi N. Chu CC, et al. Blood. 2010 May 13;115(19):3907-15. doi: 10.1182/blood-2009-09-244251. Epub 2010 Jan 28. Blood. 2010. PMID: 20110421 Free PMC article. - IgG+, CD5+ human chronic lymphocytic leukemia B cells. Production of IgG antibodies that exhibit diminished autoreactivity and IgG subclass skewing.
Wakai M, Hashimoto S, Omata M, Sthoeger ZM, Allen SL, Lichtman SM, Schulman P, Vinciguerra VP, Diamond B, Dono M, et al. Wakai M, et al. Autoimmunity. 1994;19(1):39-48. doi: 10.3109/08916939409008007. Autoimmunity. 1994. PMID: 7538331 - From normal to clonal B cells: Chronic lymphocytic leukemia (CLL) at the crossroad between neoplasia and autoimmunity.
Ghia P, Scielzo C, Frenquelli M, Muzio M, Caligaris-Cappio F. Ghia P, et al. Autoimmun Rev. 2007 Dec;7(2):127-31. doi: 10.1016/j.autrev.2007.02.014. Epub 2007 Mar 22. Autoimmun Rev. 2007. PMID: 18035322 Review. - Immunological aspects in chronic lymphocytic leukemia (CLL) development.
García-Muñoz R, Roldan Galiacho V, Llorente L. García-Muñoz R, et al. Ann Hematol. 2012 Jul;91(7):981-96. doi: 10.1007/s00277-012-1460-z. Epub 2012 Apr 12. Ann Hematol. 2012. PMID: 22526361 Free PMC article. Review.
Cited by
- Pathogenic B-cell receptor signaling in lymphoid malignancies: New insights to improve treatment.
Young RM, Phelan JD, Wilson WH, Staudt LM. Young RM, et al. Immunol Rev. 2019 Sep;291(1):190-213. doi: 10.1111/imr.12792. Immunol Rev. 2019. PMID: 31402495 Free PMC article. Review. - High-density screening reveals a different spectrum of genomic aberrations in chronic lymphocytic leukemia patients with 'stereotyped' IGHV3-21 and IGHV4-34 B-cell receptors.
Marincevic M, Cahill N, Gunnarsson R, Isaksson A, Mansouri M, Göransson H, Rasmussen M, Jansson M, Ryan F, Karlsson K, Adami HO, Davi F, Jurlander J, Juliusson G, Stamatopoulos K, Rosenquist R. Marincevic M, et al. Haematologica. 2010 Sep;95(9):1519-25. doi: 10.3324/haematol.2009.021014. Epub 2010 Apr 26. Haematologica. 2010. PMID: 20421269 Free PMC article. - From pathogenesis to treatment of chronic lymphocytic leukaemia.
Zenz T, Mertens D, Küppers R, Döhner H, Stilgenbauer S. Zenz T, et al. Nat Rev Cancer. 2010 Jan;10(1):37-50. doi: 10.1038/nrc2764. Epub 2009 Dec 3. Nat Rev Cancer. 2010. PMID: 19956173 Review. - Responsiveness of chronic lymphocytic leukemia cells to B-cell receptor stimulation is associated with low expression of regulatory molecules of the nuclear factor-κB pathway.
Meijers RWJ, Muggen AF, Leon LG, de Bie M, van Dongen JJM, Hendriks RW, Langerak AW. Meijers RWJ, et al. Haematologica. 2020 Jan;105(1):182-192. doi: 10.3324/haematol.2018.215566. Epub 2019 May 16. Haematologica. 2020. PMID: 31097630 Free PMC article. - Protective Effect of Oyster Peptides Derived From Crassostrea gigas on Intestinal Oxidative Damage Induced by Cyclophosphamide in Mice Mediated Through Nrf2-Keap1 Signaling Pathway.
Chen H, Zheng H, Li T, Jiang Q, Liu S, Zhou X, Ding Y, Xiang X. Chen H, et al. Front Nutr. 2022 May 16;9:888960. doi: 10.3389/fnut.2022.888960. eCollection 2022. Front Nutr. 2022. PMID: 35651503 Free PMC article.
References
- Rai KR. Chronic lymphocytic leukemia in the elderly population. Clin Geriatr Med. 1997;13:245–9. - PubMed
- Damle RN, et al. B-cell chronic lymphocytic leukemia cells express a surface membrane phenotype of activated, antigen-experienced B lymphocytes. Blood. 2002;99:4087–93. - PubMed
- Chiorazzi N, Ferrarini M. B cell chronic lymphocytic leukemia: lessons learned from studies of the B cell antigen receptor. Ann Rev Immunol. 2003;21:841–94. - PubMed
- Stevenson F, Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004;103:4389–95. - PubMed
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources