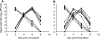Blocking interhost transmission of influenza virus by vaccination in the guinea pig model - PubMed (original) (raw)
Blocking interhost transmission of influenza virus by vaccination in the guinea pig model
Anice C Lowen et al. J Virol. 2009 Apr.
Abstract
Interventions aimed at preventing viral spread have the potential to effectively control influenza virus in all age groups, thereby reducing the burden of influenza illness. For this reason, we have examined the efficacy of vaccination in blocking the transmission of influenza viruses between guinea pigs. Three modes of immunization were compared: (i) natural infection; (ii) intramuscular administration of whole, inactivated influenza virus in 2 doses; and (iii) intranasal inoculation with live attenuated influenza virus in 2 doses. The ability of each immunization method to block the spread of a homologous (A/Panama/2007/99) H3N2 subtype and a heterologous (A/Wisconsin/67/05) H3N2 subtype influenza virus was tested. We found that previous infection through a natural route provided sterilizing immunity against both homologous and heterologous challenges; thus, no transmission to or from previously infected animals was observed. Vaccination with an inactivated influenza virus vaccine, in contrast, did not prevent guinea pigs from becoming infected upon challenge with either virus. Thus, both intranasal inoculation and exposure to an acutely infected guinea pig led to the infection of vaccinated animals. Vaccination with inactivated virus did, however, reduce viral load upon challenge and decrease the number of secondary transmission events from vaccinated animals to naïve cage mates. Vaccination with a live attenuated virus was found to be more efficacious than vaccination with inactivated virus, resulting in sterilizing immunity against homologous challenge and full protection against the transmission of the homologous and heterologous viruses to naïve contacts. In conclusion, we have shown that the guinea pig model can be used to test influenza virus vaccines and that the efficiency of transmission is a valuable readout when vaccine efficacy is evaluated.
Figures
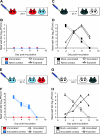
FIG. 1.
Natural infection through exposure to acutely infected guinea pigs. (A) Natural exposure 3 weeks prior to homologous challenge. Six guinea pigs were inoculated intranasally with 1,000 PFU Pan/99 virus. At 24 h postinoculation, a naïve guinea pig was placed into the same cage with each inoculated guinea pig. Animals were housed in this way for 7 days. (B) Natural exposure 3 weeks prior to heterologous challenge. Eight guinea pigs were inoculated intranasally with 1,000 PFU Pan/99 virus. At 24 h postinoculation, a naïve guinea pig was placed into the same cage with each inoculated guinea pig. Animals were housed in this way for 7 days. Dashed lines with squares represent nasal wash titers of inoculated animals; solid lines with triangles represent nasal wash titers of exposed animals.
FIG. 2.
Previously infected guinea pigs exhibit sterilizing immunity to homologous challenge. (A) Schematic representation of challenge by the intranasal route. Three previously infected guinea pigs (red) were challenged intranasally with Pan/99 virus. At 24 h postinoculation, a naïve contact animal (blue) was cocaged with each of the three inoculated guinea pigs. (B) Results of homologous challenge by the intranasal route. No virus was detected in the nasal washings of challenged guinea pigs (red squares with dashed lines), and no virus was detected in the naïve contact animals (blue triangles with solid lines). (C) Schematic representation of challenge of control guinea pigs by the intranasal route. Two control animals with no previous exposure (black) were inoculated intranasally with Pan/99 virus. At 24 h postinoculation, a naïve contact guinea pig (white) was cocaged with each of the two inoculated guinea pigs. (D) Results of Pan/99 challenge of control guinea pigs by the intranasal route. Animals with no previous exposure were productively infected through inoculation (black squares with dashed lines) and transmitted efficiently to naïve contact animals (white triangles with solid lines). (E) Schematic representation of challenge through exposure to an infected guinea pig. Three naïve guinea pigs were inoculated intranasally with Pan/99 virus. At 24 h postinoculation, each of the three acutely infected animals (blue) was placed into the same cage with one previously infected guinea pig (red). (F) Results of homologous challenge through exposure to an infected guinea pig. Intranasally infected contact animals shed high titers of virus into nasal washes (blue squares with dashed lines); however, guinea pigs with previous exposure to Pan/99 virus (red triangles with solid lines) did not become infected through contact with the infected animals. (G) Schematic representation of challenge of control animals through exposure to an infected guinea pig. Two naïve contact animals were inoculated intranasally with Pan/99 virus. At 24 h postinoculation, two control guinea pigs with no previous exposure were each placed into the same cage with one infected animal. (H) Results of control challenge through contact with an infected guinea pig. Intranasally infected contact animals shed high titers of virus into nasal washes (white squares with dashed lines), and control guinea pigs with no previous exposure to Pan/99 virus (black triangles with solid lines) became infected through contact with the infected animals.
FIG. 3.
Previously infected guinea pigs exhibit sterilizing immunity to heterologous challenge. (A) Schematic representation of challenge by the intranasal route. Four guinea pigs previously infected with Pan/99 virus (red) were challenged intranasally with Wisc/05 virus. At 24 h postinoculation, a naïve contact animal (blue) was cocaged with each of the four inoculated guinea pigs. (B) Results of heterologous challenge by the intranasal route. No virus was detected in the nasal washings of challenged guinea pigs (red squares with dashed lines), and no virus was detected in the naïve contact animals (blue triangles with solid lines). (C) Schematic representation of challenge of control guinea pigs by the intranasal route. Two control animals with no previous exposure (black) were inoculated intranasally with Wisc/05 virus. At 24 h postinoculation, a naïve contact guinea pig (white) was cocaged with each of the inoculated guinea pigs. (D) Results of Wisc/05 challenge of control guinea pigs by the intranasal route. Animals with no previous exposure were productively infected through inoculation (black squares with dashed lines). Transmission to one of two naïve contact animals was observed (white triangles with solid lines). (E) Schematic representation of challenge through exposure to an infected guinea pig. Four naïve guinea pigs were inoculated intranasally with Wisc/05 virus. At 24 h postinoculation, each acutely infected animal (blue) was placed into the same cage with one previously Pan/99 virus-infected guinea pig (red). (F) Results of heterologous challenge through exposure to an infected guinea pig. Intranasally infected contact animals shed high titers of virus into nasal washes (blue squares with dashed lines); however, the four guinea pigs with previous exposure to Pan/99 virus (red triangles with solid lines) did not become infected through contact with the infected animals. (G) Schematic representation of challenge of control animals through exposure to an infected guinea pig. Two naïve contact animals were inoculated intranasally with Wisc/05 virus. At 24 h postinoculation, two control guinea pigs with no previous exposure were each placed into the same cage with one infected animal. (H) Results of control challenge through contact with a Wisc/05-infected guinea pig. Intranasally infected contact animals shed high titers of virus into nasal washes (white squares with dashed lines), and one of the two control guinea pigs with no previous exposure (black triangles with solid lines) became infected through contact with an infected animal.
FIG. 4.
Vaccination with inactivated virus limits secondary transmission but does not protect against initial infection with homologous virus. (A) Schematic representation of challenge by the intranasal route. Four guinea pigs previously vaccinated with inactivated Pan/99 virus (red) were challenged intranasally with 1,000 PFU of Pan/99 virus. At 24 h postinoculation, a naïve contact animal (blue) was cocaged with each of the four inoculated guinea pigs. (B) Results of homologous challenge by the intranasal route. Three of four vaccinated animals (red squares with dashed lines) became infected following intranasal challenge, and one of four naïve contact animals (blue triangles with solid lines) was subsequently infected. (C) Schematic representation of challenge of mock-vaccinated guinea pigs by the intranasal route. Two previously mock-vaccinated control animals (black) were inoculated intranasally with Pan/99 virus. At 24 h postinoculation, a naïve contact guinea pig (white) was cocaged with each of the two inoculated guinea pigs. (D) Results of Pan/99 challenge of mock-vaccinated guinea pigs by the intranasal route. Mock-vaccinated guinea pigs were productively infected through inoculation (black squares with dashed lines) and transmitted efficiently to naïve contact animals (white triangles with solid lines). (E) Schematic representation of challenge through exposure to an infected guinea pig. Three naïve guinea pigs were inoculated intranasally with Pan/99 virus. At 24 h postinoculation, each acutely infected animal (blue) was placed into the same cage with one vaccinated guinea pig (red). (F) Results of homologous challenge through exposure to an infected guinea pig. Intranasally infected contact animals shed high titers of virus into nasal washes (blue squares with dashed lines); all three vaccinated guinea pigs (red triangles with solid lines) became infected through contact with the infected animals. (G) Schematic representation of challenge of mock-vaccinated animals through exposure to an infected guinea pig. Two naïve contact animals were inoculated intranasally with Pan/99 virus. At 24 h postinoculation, one mock-vaccinated guinea pig was placed into the same cage with each infected animal. (H) Results of control challenge through contact with an infected guinea pig. Intranasally infected contact animals shed high titers of virus into nasal washes (white squares with dashed lines), and both mock-vaccinated guinea pigs (black triangles with solid lines) became infected through contact with the infected animals.
FIG. 5.
Vaccination with inactivated virus limits secondary transmission but does not protect against initial infection with heterologous virus. (A) Schematic representation of challenge by the intranasal route. Four guinea pigs previously vaccinated with inactivated Pan/99 virus (red) were challenged intranasally with Wisc/05 virus. At 24 h postinoculation, a naïve contact animal (blue) was cocaged with each of the four inoculated guinea pigs. (B) Results of heterologous challenge by the intranasal route. All four previously vaccinated guinea pigs became infected upon challenge (red squares with dashed lines); however, only one of four naïve contact animals (blue triangles with solid lines) contracted infection. (C) Schematic representation of challenge of mock-vaccinated guinea pigs by the intranasal route. Two previously mock-vaccinated animals (black) were inoculated intranasally with Wisc/05 virus. At 24 h postinoculation, a naïve contact guinea pig (white) was cocaged with each of the two inoculated guinea pigs. (D) Results of Wisc/05 challenge of mock-vaccinated guinea pigs by the intranasal route. Mock-vaccinated guinea pigs were productively infected through inoculation (black squares with dashed lines). Transmission to one of two naïve contact animals was observed (white triangles with solid lines). (E) Schematic representation of heterologous challenge through exposure to an infected guinea pig. Three naïve guinea pigs were inoculated intranasally with Wisc/05 virus. At 24 h postinoculation, each acutely infected animal (blue) was placed into the same cage with one vaccinated guinea pig (red). (F) Results of heterologous challenge through exposure to an infected guinea pig. Intranasally infected contact animals shed high titers of virus into nasal washes (blue squares with dashed lines); all four animals vaccinated with killed Pan/99 virus (red triangles with solid lines) became infected through the transmission of Wisc/05. (G) Schematic representation of challenge of control animals through exposure to an infected guinea pig. Two naïve contact animals were inoculated intranasally with Wisc/05 virus. At 24 h postinoculation, one mock-vaccinated guinea pig was placed into the same cage with each infected animal. (H) Results of control challenge through contact with a Wisc/05-infected guinea pig. Intranasally infected contact animals shed high titers of virus into nasal washes (white squares with dashed lines), and both of the mock-vaccinated guinea pigs (black triangles with solid lines) became infected through contact with an infected animal.
FIG. 6.
Shedding titers of NS1-truncated LAIV following initial administration. (A) Nasal wash titers of Pan/99 HA NA:PR8 NS1-73-vaccinated animals 6 weeks prior to challenge with Pan/99 virus. (B) Nasal wash titers of Pan/99 HA NA:PR8 NS1-73-vaccinated animals 6 weeks prior to challenge with Wisc/05 virus. Solid squares show titers shed by vaccinated animals, open circles show titers obtained from mock-vaccinated controls, and open triangles show results from two sentinel guinea pigs, each housed in the same cage with two vaccinated guinea pigs.
FIG. 7.
Vaccination with an NS1-truncated LAIV provides sterilizing immunity against homologous challenge. (A) Schematic representation of challenge by the intranasal route. Four guinea pigs previously vaccinated with LAIV (red) were challenged intranasally with 1,000 PFU of Pan/99 virus. At 24 h postinoculation, a naïve contact animal (blue) was cocaged with each of the inoculated guinea pigs. (B) Results of homologous challenge by the intranasal route. No virus was detected in the nasal washings of challenged guinea pigs (red squares with dashed lines) or of the naïve contact animals (blue triangles with solid lines). (C) Schematic representation of challenge of mock-vaccinated guinea pigs by the intranasal route. Two previously mock-vaccinated control animals (black) were inoculated intranasally with Pan/99 virus. At 24 h postinoculation, a naïve contact guinea pig (white) was cocaged with each of the inoculated guinea pigs. (D) Results of Pan/99 challenge of mock-vaccinated guinea pigs by the intranasal route. Mock-vaccinated guinea pigs were productively infected through inoculation (black squares with dashed lines) and transmitted efficiently to naïve contact animals (white triangles with solid lines). (E) Schematic representation of challenge through exposure to an infected guinea pig. Four naïve guinea pigs were inoculated intranasally with Pan/99 virus. At 24 h postinoculation, each acutely infected animal (blue) was placed into the same cage with one previously vaccinated guinea pig (red). (F) Results of homologous challenge through exposure to an infected guinea pig. Intranasally infected contact animals shed high titers of virus into nasal washes (blue squares with dashed lines); no virus was detected in nasal washes of the four vaccinated animals (red triangles with solid lines). (G) Schematic representation of challenge of mock-vaccinated animals through exposure to an infected guinea pig. Two naïve contact animals were inoculated intranasally with Pan/99 virus. At 24 h postinoculation, two previously mock-vaccinated guinea pigs were each placed into the same cage with one infected animal. (H) Results of control challenge through contact with an infected guinea pig. Intranasally infected contact animals shed high titers of virus into nasal washes (white squares with dashed lines), and both mock-vaccinated guinea pigs (black triangles with solid lines) became infected through contact with the infected animals.
FIG. 8.
Vaccination with NS1-truncated LAIV provides partial protection against initial infection with, and full protection against secondary transmission of, heterologous virus. (A) Schematic representation of challenge by the intranasal route. Four guinea pigs previously vaccinated with LAIV (red) were challenged intranasally with Wisc/05 virus. At 24 h postinoculation, a naïve contact animal (blue) was cocaged with each of the four inoculated guinea pigs. (B) Results of heterologous challenge by the intranasal route. One of four vaccinated guinea pigs became infected upon challenge (red squares with dashed lines); none of the four naïve contact animals (blue triangles with solid lines) contracted infection. (C) Schematic representation of challenge of mock-vaccinated guinea pigs by the intranasal route. Two previously mock-vaccinated animals (black) were inoculated intranasally with Wisc/05 virus. At 24 h postinoculation, a naïve contact guinea pig (white) was cocaged with each of the two inoculated guinea pigs. (D) Results of Wisc/05 challenge of mock-vaccinated guinea pigs by the intranasal route. Mock-vaccinated guinea pigs were productively infected through inoculation (black squares with dashed lines), and transmission to both naïve contact animals was observed (white triangles with solid lines). (E) Schematic representation of heterologous challenge through exposure to an infected guinea pig. Four naïve guinea pigs were inoculated intranasally with Wisc/05 virus. At 24 h postinoculation, each acutely infected animal (blue) was placed into the same cage with one previously vaccinated guinea pig (red). (F) Results of heterologous challenge through exposure to an infected guinea pig. Intranasally infected contact animals shed high titers of virus into nasal washes (blue squares with dashed lines); two of four animals previously vaccinated with the LAIV (red triangles with solid lines) became infected through transmission of Wisc/05. (G) Schematic representation of challenge of control animals through exposure to an infected guinea pig. Two naïve contact animals were inoculated intranasally with Wisc/05 virus. At 24 h postinoculation, two previously mock-vaccinated guinea pigs were each placed into the same cage with one infected animal. (H) Results of control challenge through contact with a Wisc/05-infected guinea pig. Intranasally infected contact animals shed high titers of virus into nasal washes (white squares with dashed lines), and both of the mock-vaccinated guinea pigs (black triangles with solid lines) became infected through contact with an infected animal.
Similar articles
- Efficacy in pigs of inactivated and live attenuated influenza virus vaccines against infection and transmission of an emerging H3N2 similar to the 2011-2012 H3N2v.
Loving CL, Lager KM, Vincent AL, Brockmeier SL, Gauger PC, Anderson TK, Kitikoon P, Perez DR, Kehrli ME Jr. Loving CL, et al. J Virol. 2013 Sep;87(17):9895-903. doi: 10.1128/JVI.01038-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824815 Free PMC article. - Vaccines That Reduce Viral Shedding Do Not Prevent Transmission of H1N1 Pandemic 2009 Swine Influenza A Virus Infection to Unvaccinated Pigs.
Everett HE, van Diemen PM, Aramouni M, Ramsay A, Coward VJ, Pavot V, Canini L, Holzer B, Morgan S; Dynamics sLoLa Consortium; Woolhouse MEJ, Tchilian E, Brookes SM, Brown IH, Charleston B, Gilbert S. Everett HE, et al. J Virol. 2021 Jan 28;95(4):e01787-20. doi: 10.1128/JVI.01787-20. Print 2021 Jan 28. J Virol. 2021. PMID: 33268518 Free PMC article. - Poly I:C adjuvanted inactivated swine influenza vaccine induces heterologous protective immunity in pigs.
Thomas M, Wang Z, Sreenivasan CC, Hause BM, Gourapura J Renukaradhya, Li F, Francis DH, Kaushik RS, Khatri M. Thomas M, et al. Vaccine. 2015 Jan 15;33(4):542-8. doi: 10.1016/j.vaccine.2014.11.034. Epub 2014 Nov 28. Vaccine. 2015. PMID: 25437101 Free PMC article. - Efficacy of intranasal administration of a truncated NS1 modified live influenza virus vaccine in swine.
Vincent AL, Ma W, Lager KM, Janke BH, Webby RJ, García-Sastre A, Richt JA. Vincent AL, et al. Vaccine. 2007 Nov 19;25(47):7999-8009. doi: 10.1016/j.vaccine.2007.09.019. Epub 2007 Sep 29. Vaccine. 2007. PMID: 17933442 Free PMC article. Clinical Trial. - Efficacy of Heterologous Prime-Boost Vaccination with H3N2 Influenza Viruses in Pre-Immune Individuals: Studies in the Pig Model.
Chepkwony S, Parys A, Vandoorn E, Chiers K, Van Reeth K. Chepkwony S, et al. Viruses. 2020 Sep 1;12(9):968. doi: 10.3390/v12090968. Viruses. 2020. PMID: 32882956 Free PMC article.
Cited by
- Turkey versus guinea pig red blood cells: hemagglutination differences alter hemagglutination inhibition responses against influenza A/H1N1.
Ovsyannikova IG, White SJ, Albrecht RA, García-Sastre A, Poland GA. Ovsyannikova IG, et al. Viral Immunol. 2014 May;27(4):174-8. doi: 10.1089/vim.2013.0111. Epub 2014 Apr 30. Viral Immunol. 2014. PMID: 24787023 Free PMC article. - Efficacy of a parainfluenza virus 5 (PIV5)-based H7N9 vaccine in mice and guinea pigs: antibody titer towards HA was not a good indicator for protection.
Li Z, Gabbard JD, Johnson S, Dlugolenski D, Phan S, Tompkins SM, He B. Li Z, et al. PLoS One. 2015 Mar 24;10(3):e0120355. doi: 10.1371/journal.pone.0120355. eCollection 2015. PLoS One. 2015. PMID: 25803697 Free PMC article. - Transmission of pandemic H1N1 influenza virus and impact of prior exposure to seasonal strains or interferon treatment.
Steel J, Staeheli P, Mubareka S, García-Sastre A, Palese P, Lowen AC. Steel J, et al. J Virol. 2010 Jan;84(1):21-6. doi: 10.1128/JVI.01732-09. J Virol. 2010. PMID: 19828604 Free PMC article. - Transmission in the guinea pig model.
Lowen AC, Bouvier NM, Steel J. Lowen AC, et al. Curr Top Microbiol Immunol. 2014;385:157-83. doi: 10.1007/82_2014_390. Curr Top Microbiol Immunol. 2014. PMID: 25001209 Free PMC article. Review.
References
- Belshe, R. B. 2004. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 103177-185. - PubMed
- Belshe, R. B. 2007. Translational research on vaccines: influenza as an example. Clin. Pharmacol. Ther. 82745-749. - PubMed
- Belshe, R. B., K. M. Edwards, T. Vesikari, S. V. Black, R. E. Walker, M. Hultquist, G. Kemble, and E. M. Connor. 2007. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 356685-696. - PubMed
- Belshe, R. B., W. C. Gruber, P. M. Mendelman, I. Cho, K. Reisinger, S. L. Block, J. Wittes, D. Iacuzio, P. Piedra, J. Treanor, J. King, K. Kotloff, D. I. Bernstein, F. G. Hayden, K. Zangwill, L. Yan, and M. Wolff. 2000. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J. Pediatr. 136168-175. - PubMed
- Beyer, W. E. P., A. M. Palache, J. C. de Jong, and A. D. M. E. Osterhaus. 2002. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy: a meta-analysis. Vaccine 201340-1353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN266200700010C/AI/NIAID NIH HHS/United States
- HHSN 266200700010C/PHS HHS/United States
- UC19 AI062623-023/AI/NIAID NIH HHS/United States
- ST32A1007623-07/ST/OHS HRSA HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical