Intranasal administration of alpha interferon reduces seasonal influenza A virus morbidity in ferrets - PubMed (original) (raw)
Intranasal administration of alpha interferon reduces seasonal influenza A virus morbidity in ferrets
Daniela Kugel et al. J Virol. 2009 Apr.
Abstract
The type I interferon (IFN) response represents one of the first lines of defense against influenza virus infections. In this study, we assessed the protective potential of exogenous IFN-alpha against seasonal and highly pathogenic influenza viruses in ferrets. Intranasal treatment with IFN-alpha several hours before infection with the H1N1 influenza A virus strain A/USSR/90/77 reduced viral titers in nasal washes at least 100-fold compared to mock-treated controls. IFN-treated animals developed only mild and transient respiratory symptoms, and the characteristic fever peak seen in mock-treated ferrets 2 days after infection was not observed. Repeated application of IFN-alpha substantially increased the protective effect of the cytokine treatment. IFN-alpha did not increase survival after infection with the highly pathogenic H5N1 avian influenza A virus strain A/Vietnam/1203/2004. However, viral titers in nasal washes were significantly reduced at days 1 and 3 postinfection. Our study shows that intranasal application of IFN-alpha can protect ferrets from seasonal influenza viruses, which replicate mainly in the upper respiratory tract, but not from highly pathogenic influenza viruses, which also disseminate to the lung. Based on these results, a more intensive evaluation of IFN-alpha as an emergency drug against pandemic influenza A is warranted.
Figures
FIG. 1.
ftIFN-α is active in alveolar epithelial cells and upper respiratory tract of ferrets. (A) Supernatant of transfected 293T cells containing ftIFN-α or huIFN-αB/D was diluted as indicated and incubated with FtAEpCs for 16 h before the cells were lysed and subjected to Western blot analysis using Mx-specific monoclonal antibody M143. The blots were reprobed with a β-tubulin-specific antibody to verify similar loading of all lanes. Representative results from three independent experiments are shown. +, FtAEpCs treated with 104 U of _E. coli_-produced, purified huIFN-αB/D served as a positive control; nc, FtAEpCs treated with a 10−2 dilution of supernatant of mock-transfected 293T cells served as a negative control. (B) FtAEpCs were treated with different dilutions of ftIFN-α for 16 h before infection with VSV-GFP at an MOI of 1. Mock-treated FtAEpCs served as a control. Viral titers in the supernatants were determined at 15 h postinfection. Representative results from two independent experiments are shown. (C) Ferrets were treated intranasally with 300 μl of undiluted supernatant of transfected 293T cells containing ftIFN-α or supernatant of mock-transfected 293T cells (mock) at 20 and 4 h before harvesting of the lung, nasal turbinates, and cells from the trachea. A Western blot analysis of protein extracts (∼50 μg/lane) was done as in panel A. LL, lung left lobe; LR, lung right lobe; T, tracheal epithelial cells; NT, nasal turbinate.
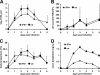
FIG. 2.
HuIFN-αB/D reduces respiratory signs after influenza A virus challenge. Groups of six animals were treated intranasally with 107 U of _E. coli_-produced IFN-αB/D in a volume of 250 μl of supernatant at 20 and 4 h before infection and at 24 h post-intranasal infection with 105 TCID50 of the H3N2 strain PC/73. The control animals were not treated. (A) Virus titers in nasal washes; (B) nasal wash (NW) cell counts; (C) body temperature. Group averages at the indicated time points are shown. Error bars represent standard deviations. (D) Respiratory signs. Sneezing, nose exudates, and congestion were graded on a 0-1-2 scale, with 0 indicating minimal deviation of the physiologic state; 1 indicating moderate nasal discharge, congestion, and/or occasional sneezing; and 2 indicating severe nasal discharge and/or labored breathing, dyspnea, and frequent sneezing. Group averages are shown.
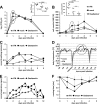
FIG. 3.
ftIFN-α protects against infection with a seasonal influenza A virus strain. Groups of six animals were treated with 300 μl of undiluted supernatant of transfected 293T cells containing ftIFN-α or 300 μl of supernatant of mock-transfected 293T cells at 20 and 4 h before infection with 105 TCID50 of H1N1 influenza A virus strain USSR/77. An additional group of animals received 2.5 mg of oseltamivir, corresponding to 2.2 to 2.5 mg/kg, twice daily for five days, starting 4 h before infection. (A) Viral titers in nasal washes at various times postinfection. Error bars represent standard deviations. (Inset) Results of statistical analysis of circled data points at 24 h postinfection. (B) Cell counts in nasal washes (NW) at various times postinfection. (Inset) Results of statistical analysis of circled data points at 48 h postinfection. (C) The breathing rate was scored using a 0-1-2 scale (score 0, <28; score 1, 28 to 36; score 2, >36). (D) Body temperature as measured by telemetry. Average values of groups of three animals are shown. The pronounced temperature drops resulted from anesthesia used for virus infection and X-ray examination of the animals, respectively. (E) Respiratory signs. Sneezing, nose exudates, and congestion were graded on a 0-1-2 scale, with 0 indicating minimal deviation of the physiologic state; 1 indicating moderate nasal discharge, congestion and/or occasional sneezing; and 2 indicating severe nasal discharge and/or labored breathing, dyspnea, and frequent sneezing. (F) Treadmill performance. Error bars indicate the standard deviation.
FIG. 4.
Repeated intranasal treatment increases protective effect of ftIFN-α. Groups of six animals were treated with 300 μl of undiluted supernatant of transfected 293T cells containing ftIFN-α or 300 μl of supernatant of mock-transfected 293T cells at 20 and 4 h before infection with 105 TCID50 of H1N1 influenza A virus strain USSR/77. Two more treatments were done at 24 and 48 h postinfection. (A) Viral titers in nasal washes. (Inset) Results of statistical analysis of circled data points. (B) Cell counts in nasal washes (NW). (Inset) Results of statistical analysis of circled data points. Error bars represent the standard deviation. (C) Body temperature as measured by telemetry. Average values of groups of three animals are shown. (D) Respiratory signs. The scoring criteria outlined in the legend to Fig. 2 were used.
FIG. 5.
ftIFN reduces infection of upper respiratory tract with H5N1 influenza A virus but does not prevent disease. Groups of six animals were treated with 300 μl of undiluted supernatant of transfected 293T cells containing ftIFN-α or 300 μl of supernatant of mock-transfected 293T cells at 20 and 4 h before infection with 104 PFU of H5N1 strain A/Vietnam/1203/2004 and observed for 12 days. (A) Body temperature. (Inset) Results of statistical analysis of circled data points. Error bars represent the standard deviation. ctr, control. (B) Viral titers in nasal washes. The detection level of the assay is indicated by a dotted line. (C) Survival. (D) Clinical signs. Each animal is represented by one square. Severity of disease is shown by color code (white, low; gray, moderate; black, severe). Clinical signs were scored daily. For respiratory signs, gray represents moderate nasal discharge and/or occasional sneezing, while black indicates severe nasal discharge and/or labored breathing and frequent sneezing. Reduced activity is represented by gray and loss of activity by black. Neurological signs were scored as moderate if either paralysis or aggressive behavior was observed and severe if both signs were present. For diarrhea, gray represents soft stool to mild diarrhea, while black indicates severe bloody diarrhea. (E) Composite score of clinical signs over the course of the disease. Posture, appetite, activity, respiration, diarrhea, and neurological signs were assessed daily and scored as outlined in Materials and Methods. The composite score represents the mean of the sum of all individual scores of each animal in the respective group. Error bars indicate standard error.
Similar articles
- Pathogenesis of 1918 pandemic and H5N1 influenza virus infections in a guinea pig model: antiviral potential of exogenous alpha interferon to reduce virus shedding.
Van Hoeven N, Belser JA, Szretter KJ, Zeng H, Staeheli P, Swayne DE, Katz JM, Tumpey TM. Van Hoeven N, et al. J Virol. 2009 Apr;83(7):2851-61. doi: 10.1128/JVI.02174-08. Epub 2009 Jan 14. J Virol. 2009. PMID: 19144714 Free PMC article. - M2SR, a novel live influenza vaccine, protects mice and ferrets against highly pathogenic avian influenza.
Hatta Y, Boltz D, Sarawar S, Kawaoka Y, Neumann G, Bilsel P. Hatta Y, et al. Vaccine. 2017 Jul 24;35(33):4177-4183. doi: 10.1016/j.vaccine.2017.06.039. Epub 2017 Jun 28. Vaccine. 2017. PMID: 28668565 Free PMC article. - Low-dose interferon Type I treatment is effective against H5N1 and swine-origin H1N1 influenza A viruses in vitro and in vivo.
Haasbach E, Droebner K, Vogel AB, Planz O. Haasbach E, et al. J Interferon Cytokine Res. 2011 Jun;31(6):515-25. doi: 10.1089/jir.2010.0071. Epub 2011 Feb 16. J Interferon Cytokine Res. 2011. PMID: 21323570 - Sequential seasonal H1N1 influenza virus infections protect ferrets against novel 2009 H1N1 influenza virus.
Carter DM, Bloom CE, Nascimento EJ, Marques ET, Craigo JK, Cherry JL, Lipman DJ, Ross TM. Carter DM, et al. J Virol. 2013 Feb;87(3):1400-10. doi: 10.1128/JVI.02257-12. Epub 2012 Oct 31. J Virol. 2013. PMID: 23115287 Free PMC article. - Characterization of the Localized Immune Response in the Respiratory Tract of Ferrets following Infection with Influenza A and B Viruses.
Carolan LA, Rockman S, Borg K, Guarnaccia T, Reading P, Mosse J, Kelso A, Barr I, Laurie KL. Carolan LA, et al. J Virol. 2015 Dec 30;90(6):2838-48. doi: 10.1128/JVI.02797-15. J Virol. 2015. PMID: 26719259 Free PMC article.
Cited by
- Oromucosal Administration of Interferon to Humans.
Beilharz MW, Cummins MJ, Bennett AL, Cummins JM. Beilharz MW, et al. Pharmaceuticals (Basel). 2010 Jan 28;3(2):323-344. doi: 10.3390/ph3020323. Pharmaceuticals (Basel). 2010. PMID: 27713254 Free PMC article. Review. - Antitumor activity of type I and type III interferons in BNL hepatoma model.
Abushahba W, Balan M, Castaneda I, Yuan Y, Reuhl K, Raveche E, de la Torre A, Lasfar A, Kotenko SV. Abushahba W, et al. Cancer Immunol Immunother. 2010 Jul;59(7):1059-71. doi: 10.1007/s00262-010-0831-3. Epub 2010 Mar 9. Cancer Immunol Immunother. 2010. PMID: 20217081 Free PMC article. - Antiviral potential of human IFN-α subtypes against influenza A H3N2 infection in human lung explants reveals subtype-specific activities.
Matos ADR, Wunderlich K, Schloer S, Schughart K, Geffers R, Seders M, Witt M, Christersson A, Wiewrodt R, Wiebe K, Barth P, Hocke A, Hippenstiel S, Hönzke K, Dittmer U, Sutter K, Rescher U, Rodionycheva S, Matera N, Ludwig S, Brunotte L. Matos ADR, et al. Emerg Microbes Infect. 2019;8(1):1763-1776. doi: 10.1080/22221751.2019.1698271. Emerg Microbes Infect. 2019. PMID: 31826721 Free PMC article. - Alphavirus replicon-based adjuvants enhance the immunogenicity and effectiveness of Fluzone ® in rhesus macaques.
Carroll TD, Matzinger SR, Barro M, Fritts L, McChesney MB, Miller CJ, Johnston RE. Carroll TD, et al. Vaccine. 2011 Jan 29;29(5):931-40. doi: 10.1016/j.vaccine.2010.11.024. Epub 2010 Nov 25. Vaccine. 2011. PMID: 21111777 Free PMC article. - Oral delivery of oligomeric procyanidins in Apple Poly® enhances type I IFN responses in vivo.
Snyder DT, Robison A, Kemoli S, Kimmel E, Holderness J, Jutila MA, Hedges JF. Snyder DT, et al. J Leukoc Biol. 2014 May;95(5):841-847. doi: 10.1189/jlb.0513296. Epub 2014 Jan 13. J Leukoc Biol. 2014. PMID: 24421266 Free PMC article. Clinical Trial.
References
- Beilharz, M. W., J. M. Cummins, and A. L. Bennett. 2007. Protection from lethal influenza virus challenge by oral type 1 interferon. Biochem. Biophys. Res. Commun. 355740-744. - PubMed
- Boltz, D. A., J. E. Rehg, J. McClaren, R. G. Webster, and E. A. Govorkova. 2008. Oseltamivir prophylactic regimens prevent H5N1 influenza morbidity and mortality in a ferret model. J. Infect. Dis. 1971315-1323. - PubMed
- Borecky, L., and N. Fuchsberger. 1983. Interferon as therapeutic agent. Acta Virol. 27359-370. - PubMed
- Enserink, M. 2004. Influenza. W. H. O. adds more “1918” to pandemic predictions. Science 3062025. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical