Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure - PubMed (original) (raw)
. 2009 Mar 3;106(9):3125-30.
doi: 10.1073/pnas.0813131106. Epub 2009 Feb 6.
James A Galbraith, Catherine G Galbraith, Jennifer Lippincott-Schwartz, Jennifer M Gillette, Suliana Manley, Rachid Sougrat, Clare M Waterman, Pakorn Kanchanawong, Michael W Davidson, Richard D Fetter, Harald F Hess
Affiliations
- PMID: 19202073
- PMCID: PMC2637278
- DOI: 10.1073/pnas.0813131106
Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure
Gleb Shtengel et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Understanding molecular-scale architecture of cells requires determination of 3D locations of specific proteins with accuracy matching their nanometer-length scale. Existing electron and light microscopy techniques are limited either in molecular specificity or resolution. Here, we introduce interferometric photoactivated localization microscopy (iPALM), the combination of photoactivated localization microscopy with single-photon, simultaneous multiphase interferometry that provides sub-20-nm 3D protein localization with optimal molecular specificity. We demonstrate measurement of the 25-nm microtubule diameter, resolve the dorsal and ventral plasma membranes, and visualize the arrangement of integrin receptors within endoplasmic reticulum and adhesion complexes, 3D protein organization previously resolved only by electron microscopy. iPALM thus closes the gap between electron tomography and light microscopy, enabling both molecular specification and resolution of cellular nanoarchitecture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Schematics and operating principle of multiphase interferometric microscope illustrating how Z-position is resolved. (A and B) Schematic of the single-photon multiphase fluorescence interferometer. A point source with z-position δ emits a single photon both upwards and downwards. These 2 beams (color coded as red and green in B) interfere in a special 3-way beam splitter. (C) The self-interfered photon propagates to the 3 color-coded CCD cameras with amplitudes that oscillate 120° out of phase as indicated.
Fig. 2.
X, Y, Z resolution of iPALM and its dependence on source brightness, illustrating iPALM's sub-20-nm 3D resolution with endogenous FP labels. (A) A histogram of experimentally determined positions from repeatedly sampling (25,000 frames) a source where ≈1,200 photons are detected per frame from ≈1,500 photons emitted into a 4π solid angle. (B) Axial (solid red circles) and lateral (solid blue squares) resolution of iPALM determined from FWHM of localization of Au beads of different brightness. Note that the positional FWHM number is 2.4 times larger than σ the variance that is also used to characterize resolution. Axial (empty red circles) and lateral (empty blue squares) resolution of the defocusing method determined from FWHM of localized position of Au beads of different brightness. Large ovals indicate approximately the published results for axial (red) and lateral (blue) resolutions of 3D STORM (2) and BP PALM (3). The typical photon output of fluorescent protein tags and synthetic fluorophores are depicted as pink and green gradients. Also shown (horizontal dashed lines) are addition uncertainties resulting from the displacement between the target protein and the fluorescent probes for different imaging methods.
Fig. 3.
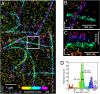
Superresolution iPALM image of microtubules in a PtK1 cell expressing human α-tubulin fused to _m_-KikGR, rendered with z axis color-coding. (A) Large area overview. (B and C) Zoom-in of the area bound by the white box in X–Y (B) and Z–Y (C) projections (_z_-scale is magnified 5×). (D) Histogram of _z_-positions of molecules in the boxed region. Each microtubule has a FWHM of 30 nm, and the separation distance of 70 nm between the cyan and purple microtubules is easily resolved.
Fig. 4.
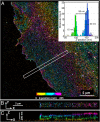
Superresolution iPALM image of COS7 cell expressing the membrane protein VSVG fused to td-EosFP, rendered with z axis color-coding. (A) Large area overview. (B and C) Area outlined in white is enlarged in B and shown in C as a z cross-section. Shown in the Inset is the histogram of vertical distribution of fluorescent molecules in the area limited by the red rectangle.
Fig. 5.
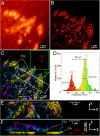
U2OS cell expressing td-EosFP-αv-integrin. (A–C) Widefield (A) and PALM (B) images, and z color-coded iPALM image (C). The coverslip surface (CS), focal adhesion (FA), cell plasma membrane (PM), and endoplasmic reticulum (ER) can be identified. (D) _Z_-position histogram of area limited by the green box in C with peaks corresponding to CS and FA. (E and F) X–Y (E) and X–Z (F) projections of the area bound by the white box in C. Z-scale is magnified by 4 in F.
Similar articles
- Three-dimensional Super Resolution Microscopy of F-actin Filaments by Interferometric PhotoActivated Localization Microscopy (iPALM).
Wang Y, Kanchanawong P. Wang Y, et al. J Vis Exp. 2016 Dec 1;(118):54774. doi: 10.3791/54774. J Vis Exp. 2016. PMID: 27929472 Free PMC article. - Imaging cellular ultrastructure by PALM, iPALM, and correlative iPALM-EM.
Shtengel G, Wang Y, Zhang Z, Goh WI, Hess HF, Kanchanawong P. Shtengel G, et al. Methods Cell Biol. 2014;123:273-94. doi: 10.1016/B978-0-12-420138-5.00015-X. Methods Cell Biol. 2014. PMID: 24974033 - 3D super-resolution imaging by localization microscopy.
Magenau A, Gaus K. Magenau A, et al. Methods Mol Biol. 2015;1232:123-36. doi: 10.1007/978-1-4939-1752-5_11. Methods Mol Biol. 2015. PMID: 25331133 - About samples, giving examples: Optimized Single Molecule Localization Microscopy.
Jimenez A, Friedl K, Leterrier C. Jimenez A, et al. Methods. 2020 Mar 1;174:100-114. doi: 10.1016/j.ymeth.2019.05.008. Epub 2019 May 10. Methods. 2020. PMID: 31078795 Review. - Super-resolution microscopy demystified.
Schermelleh L, Ferrand A, Huser T, Eggeling C, Sauer M, Biehlmaier O, Drummen GPC. Schermelleh L, et al. Nat Cell Biol. 2019 Jan;21(1):72-84. doi: 10.1038/s41556-018-0251-8. Epub 2019 Jan 2. Nat Cell Biol. 2019. PMID: 30602772 Review.
Cited by
- Cytokinetic contractile ring structural progression in an early embryo: positioning of scaffolding proteins, recruitment of α-actinin, and effects of myosin II inhibition.
Henson JH, Reyes G, Lo NT, Herrera K, McKim QW, Herzon HY, Galvez-Ceron M, Hershey AE, Kim RS, Shuster CB. Henson JH, et al. Front Cell Dev Biol. 2024 Sep 27;12:1483345. doi: 10.3389/fcell.2024.1483345. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39398481 Free PMC article. - Universal inverse modeling of point spread functions for SMLM localization and microscope characterization.
Liu S, Chen J, Hellgoth J, Müller LR, Ferdman B, Karras C, Xiao D, Lidke KA, Heintzmann R, Shechtman Y, Li Y, Ries J. Liu S, et al. Nat Methods. 2024 Jun;21(6):1082-1093. doi: 10.1038/s41592-024-02282-x. Epub 2024 Jun 3. Nat Methods. 2024. PMID: 38831208 - Coumarin as a general switching auxiliary to prepare photochromic and spontaneously blinking fluorophores.
Jradi FM, English BP, Brown TA, Aaron J, Khuon S, Galbraith JA, Galbraith CG, Lavis LD. Jradi FM, et al. bioRxiv [Preprint]. 2024 May 12:2024.05.12.593749. doi: 10.1101/2024.05.12.593749. bioRxiv. 2024. PMID: 38766036 Free PMC article. Preprint. - Focal adhesions contain three specialized actin nanoscale layers.
Kumari R, Ven K, Chastney M, Kokate SB, Peränen J, Aaron J, Kogan K, Almeida-Souza L, Kremneva E, Poincloux R, Chew TL, Gunning PW, Ivaska J, Lappalainen P. Kumari R, et al. Nat Commun. 2024 Mar 21;15(1):2547. doi: 10.1038/s41467-024-46868-7. Nat Commun. 2024. PMID: 38514695 Free PMC article. - Cryosectioning-enabled super-resolution microscopy for studying nuclear architecture at the single protein level.
Stein J, Ericsson M, Nofal M, Magni L, Aufmkolk S, McMillan RB, Breimann L, Herlihy CP, Lee SD, Willemin A, Wohlmann J, Arguedas-Jimenez L, Yin P, Pombo A, Church GM, Wu CK. Stein J, et al. bioRxiv [Preprint]. 2024 Feb 5:2024.02.05.576943. doi: 10.1101/2024.02.05.576943. bioRxiv. 2024. PMID: 38370628 Free PMC article. Preprint.
References
- Juette MF, et al. Three-dimensional sub-100 nm resolution fluorescence microscopy of thick samples. Nat Methods. 2008;5(6):527–529. - PubMed
- Schmidt R, et al. Spherical nanosized focal spot unravels the interior of cells. Nat Methods. 2008;5(6):539–544. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous