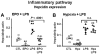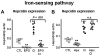Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli - PubMed (original) (raw)
Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli
Hua Huang et al. Blood. 2009.
Abstract
Hepcidin, a key regulator of iron metabolism, is a small antimicrobial peptide produced by the liver that regulates intestinal iron absorption and iron recycling by macrophages. Hepcidin is stimulated when iron stores increase and during inflammation and, conversely, is inhibited by hypoxia and augmented erythropoiesis. In many pathologic situations, such as in the anemia of chronic disease (ACD) and iron-loading anemias, several of these factors may be present concomitantly and may generate opposing signaling to regulate hepcidin expression. Here, we address the question of dominance among the regulators of hepcidin expression. We show that erythropoiesis drive, stimulated by erythropoietin but not hypoxia, down-regulates hepcidin in a dose-dependent manner, even in the presence of lipopolysaccharide (LPS) or dietary iron-loading, which may act additively. These effects are mediated through down-regulation of phosphorylation of Stat3 triggered by LPS and of Smad1/5/8 induced by iron. In conclusion, hepcidin expression levels in the presence of opposing signaling are determined by the strength of the individual stimuli rather than by an absolute hierarchy among signaling pathways. Our findings also suggest that erythropoietic drive can inhibit both inflammatory and iron-sensing pathways, at least in part, via the suppression of STAT3 and SMAD4 signaling in vivo.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
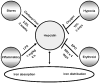
Figure 1. Factors affecting mRNA hepcidin expression in the liver
Hepcidin levels are regulated by iron levels (store regulator), immune mediators (inflammatory regulator), hypoxia (hypoxia regulator), and erythropoietic demand (erythroid regulator). Pointed arrows indicate up-regulation of hepcidin, and blunt arrows inhibition of its mRNA expression. For each regulator, the treatments used in this study to stimulate or suppress hepcidin expression are shown.
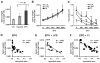
Figure 2. Erythropoietin but not hypoxia inhibits hepcidin induction through the inflammatory pathway
(A) Hepcidin mRNA levels in the liver of mice treated with saline (control [CTL]), EPO, LPS, and mice with combined treatments (EPO + LPS). (B) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), mice subjected to 10% oxygen (hypoxia [Hpx]), LPS, and mice with combined treatments (Hpx + LPS). Hepatic hepcidin expression was quantified by real-time RT-PCR and normalized to β-actin. The hepcidin/β-actin ratios are shown, each symbol representing 1 mouse. Statistical analysis was performed by 1-way ANOVA; **P < .001 for comparison with control mice. n.s. indicates not significant.
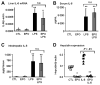
Figure 3. EPO inhibits LPS-mediated hepcidin induction independently of IL-6 production
(A) IL-6 mRNA levels in liver, (B) IL-6 levels in serum, and (C) intrahepatic IL-6 protein levels in mice treated with saline (CTL), EPO, LPS, and mice with combined treatments (EPO + LPS). (D) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), EPO, mouse recombinant IL-6 (IL-6), and mice with combined treatments (EPO + IL-6). IL-6 and hepcidin mRNA levels were quantified by real-time RT-PCR and normalized to β-actin. The IL-6/β-actin and hepcidin/β-actin ratios are shown. IL-6 protein levels were measured by ELISA. Statistical analysis was performed by 1-way ANOVA; *P < .01 and **P < .001 for comparison with control mice. Data are presented as means plus or minus SD with n = 6 mice per group in panels A through C and as individual mice in panel D. n.s. indicates not significant.
Figure 4. Stat3 phosphorylation induced by LPS is partially inhibited by EPO but not by hypoxia
(A, C) Liver nuclear extracts from mice treated with saline (CTL), EPO, hypoxia, LPS, and mice with combined treatments (EPO + LPS and hypoxia + LPS) were analyzed by Western blotting with an antibody to phosphorylated Stat3 and total Stat3. Blots were stripped and reprobed with an antibody to β-actin as loading control. A representative Western blot is shown. Lane “+” is a positive control consisting of total cell extracts from serum-starved HeLa cells prepared with interferon-α treatment. (B, D) Quantification of chemiluminescence to calculate the ratio of phosphorylated Stat3 relative to β-actin (pStat3/β-actin). This experiment was repeated twice, and the combined results are shown as means plus or minus SD with n = 7. Statistical analysis was performed by 1-way ANOVA; *P < .01, **P < .001; and ***P < .0001 for comparison with control mice. n.s. indicates not significant.
Figure 5. EPO but not hypoxia inhibits hepcidin induction through the iron-sensing pathway
(A) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), EPO, LPS, carbonyl iron–supplemented diet (2.5% CI), and mice with combined treatments (EPO + CI). (B) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), mice subjected to 10% oxygen (Hpx), 2.5% CI, and mice with combined treatments (Hpx + CI). Hepatic hepcidin expression was quantified by real-time RT-PCR and normalized to β-actin. The hepcidin/β-actin ratios are shown, each symbol representing one mouse. Statistical analysis was performed by 1-way ANOVA; **P < .001 for comparison with control mice. n.s. indicates not significant.
Figure 6. Smad1/5/8 phosphorylation induced by dietary iron-loading is partially inhibited by erythropoietin, but not by hypoxia
(A, C) Liver nuclear extracts from mice treated with saline (CTL), EPO, 2.5% CI, hypoxia, and mice with combined treatments (EPO + CI and Hypoxia + CI) were analyzed by Western blotting with an antibody to phosphorylated Smad1/5/8 and total Smad1/5/8. Blots were stripped and reprobed with an antibody to β-actin as loading control. A representative Western blot is shown. (B, D) Quantification of chemiluminescence to calculate the ratio of phosphorylated Smad1/5/8 relative to β-actin (pSmad/β-actin). This experiment was repeated twice, and the combined results are shown as means plus or minus SD with n = 7. Statistical analysis was performed by 1-way ANOVA; *P < .01; and **P < .001 for comparison with control mice. n.s. indicates not significant.
Figure 7. Relationship between erythropoiesis rate and hepatic hepcidin expression
(A) Spleen weight–body weight ratio in control, hypoxic, and EPO-treated mice (50 U for 4 days). (B) Spleen weight–body weight ratio and (C) hepatic hepcidin expression in mice treated with increasing amounts of EPO (total dosage over 4 days is shown) alone (CTL) and in combination with LPS or CI-supplemented diet (CI). (A–C) Data are presented as means plus or minus SD with n = 5 to 6 mice per group. Statistical analysis was performed by 1-way ANOVA; *P < .01 and **P < .001 for comparison with control mice. (D–F) Negative correlation between spleen weight–body weight ratio and hepatic hepcidin expression in mice treated with increasing amounts of EPO: (D) alone (EPO); (E) in combination with LPS (EPO + LPS); and (F) in combination with CI-supplemented diet (EPO + CI). Hepatic hepcidin expression was quantified by real-time RT-PCR and normalized to β-actin. The hepcidin/β-actin ratios are shown.
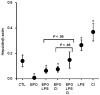
Figure 8. Additive effect of LPS and dietary iron on EPO-mediated hepcidin suppression
Hepatic hepcidin expression in control mice (CTL), mice treated with EPO alone (50 U for 4 days; EPO) and in combination with LPS (EPO + LPS), with CI-supplemented diet (EPO + CI), with both LPS and CI (EPO + LPS + CI), with LPS alone (LPS), and CI-supplemented diet alone (CI). Data are presented as means plus or minus SD with n = 6 mice per group. Statistical analysis was performed by 1-way ANOVA; *P < .05 for comparison with control mice.
Similar articles
- Iron regulation of hepcidin despite attenuated Smad1,5,8 signaling in mice without transferrin receptor 2 or Hfe.
Corradini E, Rozier M, Meynard D, Odhiambo A, Lin HY, Feng Q, Migas MC, Britton RS, Babitt JL, Fleming RE. Corradini E, et al. Gastroenterology. 2011 Nov;141(5):1907-14. doi: 10.1053/j.gastro.2011.06.077. Epub 2011 Jul 13. Gastroenterology. 2011. PMID: 21745449 Free PMC article. - Hepcidin regulation by BMP signaling in macrophages is lipopolysaccharide dependent.
Wu X, Yung LM, Cheng WH, Yu PB, Babitt JL, Lin HY, Xia Y. Wu X, et al. PLoS One. 2012;7(9):e44622. doi: 10.1371/journal.pone.0044622. Epub 2012 Sep 13. PLoS One. 2012. PMID: 23028567 Free PMC article. - Pathways for the regulation of hepcidin expression in anemia of chronic disease and iron deficiency anemia in vivo.
Theurl I, Schroll A, Nairz M, Seifert M, Theurl M, Sonnweber T, Kulaksiz H, Weiss G. Theurl I, et al. Haematologica. 2011 Dec;96(12):1761-9. doi: 10.3324/haematol.2011.048926. Epub 2011 Aug 22. Haematologica. 2011. PMID: 21859731 Free PMC article. - Regulation of systemic iron homeostasis.
Finberg KE. Finberg KE. Curr Opin Hematol. 2013 May;20(3):208-14. doi: 10.1097/MOH.0b013e32835f5a47. Curr Opin Hematol. 2013. PMID: 23426198 Review. - Hepcidin and iron homeostasis.
Ganz T, Nemeth E. Ganz T, et al. Biochim Biophys Acta. 2012 Sep;1823(9):1434-43. doi: 10.1016/j.bbamcr.2012.01.014. Epub 2012 Jan 26. Biochim Biophys Acta. 2012. PMID: 22306005 Free PMC article. Review.
Cited by
- Effect of iron overload and iron deficiency on liver hemojuvelin protein.
Krijt J, Frýdlová J, Kukačková L, Fujikura Y, Přikryl P, Vokurka M, Nečas E. Krijt J, et al. PLoS One. 2012;7(5):e37391. doi: 10.1371/journal.pone.0037391. Epub 2012 May 18. PLoS One. 2012. PMID: 22629388 Free PMC article. - Severe iron deficiency blunts the response of the iron regulatory gene Hamp and pro-inflammatory cytokines to lipopolysaccharide.
Darshan D, Frazer DM, Wilkins SJ, Anderson GJ. Darshan D, et al. Haematologica. 2010 Oct;95(10):1660-7. doi: 10.3324/haematol.2010.022426. Epub 2010 May 29. Haematologica. 2010. PMID: 20511664 Free PMC article. - Hepicidin and its role in iron metabolism.
Choudhry VP. Choudhry VP. Indian J Pediatr. 2010 Jul;77(7):787-8. doi: 10.1007/s12098-010-0098-x. Epub 2010 Jun 29. Indian J Pediatr. 2010. PMID: 20589458 No abstract available. - Alcohol's Dysregulation of Maternal-Fetal IL-6 and p-STAT3 Is a Function of Maternal Iron Status.
Saini N, Helfrich KK, Kwan STC, Huebner SM, Abazi J, Flentke GR, Blohowiak SE, Kling PJ, Smith SM. Saini N, et al. Alcohol Clin Exp Res. 2019 Nov;43(11):2332-2343. doi: 10.1111/acer.14200. Epub 2019 Oct 8. Alcohol Clin Exp Res. 2019. PMID: 31524964 Free PMC article. - Hepcidin mimetics in polycythemia vera: resolving the irony of iron deficiency and erythrocytosis.
Handa S, Ginzburg Y, Hoffman R, Kremyanskaya M. Handa S, et al. Curr Opin Hematol. 2023 Mar 1;30(2):45-52. doi: 10.1097/MOH.0000000000000747. Epub 2022 Dec 29. Curr Opin Hematol. 2023. PMID: 36728649 Free PMC article. Review.
References
- Aisen P, Wessling-Resnick M, Leibold EA. Iron metabolism. Curr Opin Chem Biol. 1999;3:200–206. - PubMed
- Finch C. Regulators of iron balance in humans. Blood. 1994;84:1697–1702. - PubMed
- Osterloh KR, Simpson RJ, Snape S, Peters TJ. Intestinal iron absorption and mucosal transferrin in rats subjected to hypoxia. Blut. 1987;55:421–431. - PubMed
- Hershko C, Cook JD, Finch CA. Storage iron kinetics, VI: the effect of inflammation on iron exchange in the rat. Br J Haematol. 1974;28:67–75. - PubMed
- Raja KB, Simpson RJ, Pippard MJ, Peters TJ. In vivo studies on the relationship between intestinal iron (Fe3+) absorption, hypoxia and erythropoiesis in the mouse. Br J Haematol. 1988;68:373–378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous