De novo deletion 17p13.1 chronic lymphocytic leukemia shows significant clinical heterogeneity: the M. D. Anderson and Mayo Clinic experience - PubMed (original) (raw)
Multicenter Study
De novo deletion 17p13.1 chronic lymphocytic leukemia shows significant clinical heterogeneity: the M. D. Anderson and Mayo Clinic experience
Constantine S Tam et al. Blood. 2009.
Abstract
To determine the clinical fate of patients with de novo deletion 17p13.1 (17p-) chronic lymphocytic leukemia (CLL), we retrospectively studied the outcome of 99 treatment-naive 17p- CLL patients from the M. D. Anderson Cancer Center (n = 64) and the Mayo Clinic (n = 35). Among 67 asymptomatic patients followed for progression, 53% developed CLL requiring treatment over 3 years. Patients who had not progressed by 18 months subsequently had stable disease, with 3 of 19 patients progressing after follow-up of up to 70 months. Risk factors for progressive disease were Rai stage of 1 or higher and unmutated immunoglobulin variable region heavy chain (IgVH). The overall survival rate was 65% at 3 years. Rai stage 1 or higher, unmutated IgVH, and 17p- in 25% or more of nuclei were adverse factors for survival. The 3-year survival rates of patients with 1 or fewer, 2, and 3 of these factors were 95%, 74%, and 22%, respectively (P < .001). Response rates to therapy with rituximab (n = 6); purine analogues and rituximab (n = 25); and purine analogues, rituximab, and alemtuzumab (n = 16) combinations were 50%, 72%, and 81%, respectively. Patients with 17p- CLL exhibit clinical heterogeneity, with some patients experiencing an indolent course. Survival can be predicted using clinical and biologic characteristics.
Figures
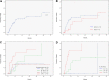
Figure 1
Progression to therapy requirement for asymptomatic patients after diagnosis of 17p− CLL. (A) The 3 year progression rate to therapy requirement was 53% for the study cohort. (B) Progression to therapy requirement by institution. The 3-year progression rate to therapy requirement was 56% for patients from MDACC and 47% for patients from the Mayo Clinic (P = .17). (C) Progression to therapy requirement, effect of clone size. Patients with 75% or more nuclei with 17p− were at increased risk of progression (79% at 3 years vs 40% for the remaining patients, P = .001). (D) Model for progression to first therapy. Risk factors were Rai stage 1 or higher, and unmutated IgVH gene. Patients with 0, 1, and 2 risk factors had 3-year progression rates of 0%, 54%, and 100%, respectively (P < .001). Thirteen patients were not assessable due to missing data on IgVH status.
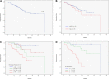
Figure 2
Survival after 17p− CLL diagnosis. (A) The rate of survival 3 years from the date of FISH diagnosis was 65%. (B) Survival after 17p− CLL diagnosis by institution. The rate of survival 3 years from the date of FISH diagnosis was 71% for patients from MDACC and 56% for patients from the Mayo Clinic (P = .43). (C) Survival after 17p− CLL diagnosis, effect of clone size. Survival was most favorable in patients with less than 25% deleted nuclei (3-year survival rate, 92%), intermediate in patients with 25% to 74% deleted nuclei (3-year survival rate, 67%), and least favorable in patients with 75% or more deleted nuclei (3-year survival rate, 40%; P = .001). (D) Model for survival after 17p− CLL diagnosis. Risk factors were Rai stage 1 or higher, unmutated IgVH gene, and 17p loss in ≥ 25% of nuclei. Patients with 1 or fewer, 2, and 3 risk factors had 3-year survival rates of 95%, 74%, and 22%, respectively (P < .001). Sixteen patients were not assessable due to missing data on IgVH status.
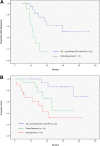
Figure 3
Time to progression (TTP) after first therapy. (A) Patients with a complete response (CR), unconfirmed CR, or nodular partial response (Nod PR) experienced a significantly better TTP (51% at 3 years) than patients with a partial response (median TTP 14 months, P = .001 vs CR/Nod PR). Three patients with a partial response were not assessable due to inadequate follow-up of disease status. **(**B) Survival after first therapy. Patients with a complete response (CR), unconfirmed CR, or nodular partial response (Nod PR) experienced significantly better survival (86% at 3 years) than patients with a partial response (median survival 28 months, P = .05 vs CR/Nod PR) or patients with no response to treatment (median survival 20 months; P = .001 vs CR/Nod PR; P = .13 vs partial response).
Similar articles
- Chemoimmunotherapy may overcome the adverse prognostic significance of 11q deletion in previously untreated patients with chronic lymphocytic leukemia.
Tsimberidou AM, Tam C, Abruzzo LV, O'Brien S, Wierda WG, Lerner S, Kantarjian HM, Keating MJ. Tsimberidou AM, et al. Cancer. 2009 Jan 15;115(2):373-80. doi: 10.1002/cncr.23993. Cancer. 2009. PMID: 19117034 Free PMC article. - Early treatment of high-risk chronic lymphocytic leukemia with alemtuzumab and rituximab.
Zent CS, Call TG, Shanafelt TD, Tschumper RC, Jelinek DF, Bowen DA, Secreto CR, Laplant BR, Kabat BF, Kay NE. Zent CS, et al. Cancer. 2008 Oct 15;113(8):2110-8. doi: 10.1002/cncr.23824. Cancer. 2008. PMID: 18759253 Free PMC article. Clinical Trial. - Cyclophosphamide, fludarabine, alemtuzumab, and rituximab as salvage therapy for heavily pretreated patients with chronic lymphocytic leukemia.
Badoux XC, Keating MJ, Wang X, O'Brien SM, Ferrajoli A, Faderl S, Burger J, Koller C, Lerner S, Kantarjian H, Wierda WG. Badoux XC, et al. Blood. 2011 Aug 25;118(8):2085-93. doi: 10.1182/blood-2011-03-341032. Epub 2011 Jun 13. Blood. 2011. PMID: 21670470 Free PMC article. Clinical Trial. - [News in therapeutic management of chronic lymphoid leukemia].
Michallet AS, Salles G, Coiffier B, Michallet M. Michallet AS, et al. Bull Cancer. 2005 Mar;92(3):249-56. Bull Cancer. 2005. PMID: 15820919 Review. French. - Chronic lymphocytic leukemia: current and emerging treatment approaches.
Kay NE, Rai KR, O'Brien S. Kay NE, et al. Clin Adv Hematol Oncol. 2006 Nov;4(11 Suppl 22):1-10; quiz 11-2. Clin Adv Hematol Oncol. 2006. PMID: 17143256 Review.
Cited by
- Prognostic significance of translocations in the presence of mutated IGHV and of cytogenetic complexity at diagnosis of chronic lymphocytic leukemia.
Heerema NA, Muthusamy N, Zhao Q, Ruppert AS, Breidenbach H, Andritsos LA, Grever MR, Maddocks KJ, Woyach J, Awan F, Long M, Gordon A, Coombes C, Byrd JC. Heerema NA, et al. Haematologica. 2021 Jun 1;106(6):1608-1615. doi: 10.3324/haematol.2018.212571. Haematologica. 2021. PMID: 32414849 Free PMC article. - Del17p does not always significantly influence the survival of B-cell chronic lymphoproliferative disorders.
Yi S, Li Z, Zou D, Xiong W, Li H, Cui R, Li C, Yan Y, Liu W, Lv R, Yu Z, Chen W, Xu Y, An G, Wang H, Ru K, Cheng T, Wang J, Qiu L. Yi S, et al. Oncotarget. 2017 Dec 15;9(3):3353-3364. doi: 10.18632/oncotarget.23261. eCollection 2018 Jan 9. Oncotarget. 2017. PMID: 29423051 Free PMC article. - Allogeneic stem cell transplant in patients with chronic lymphocytic leukemia with 17p deletion: consult-transplant versus consult- no-transplant analysis.
Poon ML, Fox PS, Samuels BI, O'Brien S, Jabbour E, Hsu Y, Gulbis A, Korbling M, Champlin R, Abruzzo LV, Bassett RL, Khouri IF. Poon ML, et al. Leuk Lymphoma. 2015 Mar;56(3):711-5. doi: 10.3109/10428194.2014.930848. Epub 2014 Aug 4. Leuk Lymphoma. 2015. PMID: 24913509 Free PMC article. - CD34+ cell of origin for immunoglobulin heavy chain variable region unmutated, but not mutated, chronic lymphocytic leukemia.
Perkins B, Showel M, Schoch L, Imus PH, Karantanos T, Yonescu R, Morsberger L, Ghiaur G, Gladstone DE, Jones RJ. Perkins B, et al. Leuk Lymphoma. 2022 Jul;63(7):1617-1623. doi: 10.1080/10428194.2022.2038375. Epub 2022 Mar 27. Leuk Lymphoma. 2022. PMID: 35343368 Free PMC article. - Modern treatment in chronic lymphocytic leukemia: impact on survival and efficacy in high-risk subgroups.
Cuneo A, Cavazzini F, Ciccone M, Daghia G, Sofritti O, Saccenti E, Negrini M, Rigolin GM. Cuneo A, et al. Cancer Med. 2014 Jun;3(3):555-64. doi: 10.1002/cam4.226. Epub 2014 Mar 19. Cancer Med. 2014. PMID: 24648042 Free PMC article.
References
- Geisler CH, Philip P, Christensen BE, et al. In B-cell chronic lymphocytic leukaemia chromosome 17 abnormalities and not trisomy 12 are the single most important cytogenetic abnormalities for the prognosis: a cytogenetic and immunophenotypic study of 480 unselected newly diagnosed patients. Leuk Res. 1997;21:1011–1023. - PubMed
- Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. - PubMed
- Dohner H, Fischer K, Bentz M, et al. p53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood. 1995;85:1580–1589. - PubMed
- Byrd JC, Smith L, Hackbarth ML, et al. Interphase cytogenetic abnormalities in chronic lymphocytic leukemia may predict response to rituximab. Cancer Res. 2003;63:36–38. - PubMed
- Stilgenbauer S, Krober A, Busch R, et al. 17p Deletion predicts for inferior overall survival after fludarabine-based first line therapy in chronic lymphocytic leukemia: first analysis of genetics in the CLL4 Trial of the GCLLSG [abstract]. Blood. 2005;106 Abstract 715.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources