Amphiregulin mediates self-renewal in an immortal mammary epithelial cell line with stem cell characteristics - PubMed (original) (raw)
Amphiregulin mediates self-renewal in an immortal mammary epithelial cell line with stem cell characteristics
Brian W Booth et al. Exp Cell Res. 2010.
Abstract
Amphiregulin (AREG), a ligand for epidermal growth factor receptor, is required for mammary gland ductal morphogenesis and mediates estrogen actions in vivo, emerging as an essential growth factor during mammary gland growth and differentiation. The COMMA-D beta-geo (CDbetageo) mouse mammary cell line displays characteristics of normal mammary progenitor cells including the ability to regenerate a mammary gland when transplanted into the cleared fat pad of a juvenile mouse, nuclear label retention, and the capacity to form anchorage-independent mammospheres. We demonstrate that AREG is essential for formation of floating mammospheres by CDbetageo cells and that the mitogen activated protein kinase signaling pathway is involved in AREG-mediated mammosphere formation. Addition of exogenous AREG promotes mammosphere formation in cells where AREG expression is knocked down by siRNA and mammosphere formation by AREG(-/-) mammary epithelial cells. AREG knockdown inhibits mammosphere formation by duct-limited mammary progenitor cells but not lobule-limited mammary progenitor cells. These data demonstrate AREG mediates the function of a subset of mammary progenitor cells in vitro.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
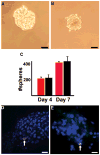
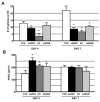
Figure 1
Mammospheres form from wild type mammary glands and CDβgeo cells. A) Mammosphere that formed from cells dissociated from a virgin wild type Balb/c mammary gland after 4 days in culture. B) Mammosphere that formed from CDβgeo cells after 4 days in culture. C) Graph depicting the number of spheres formed from seeding 1000 cells/ml after 4 and 7 days in culture from wild type Balb/c mammary glands (red bars) or CDβgeo cells (black bars). D) Mammosphere containing a nuclear label (5BrdU, pink, arrow) retaining cell following a 12 day chase period indicating asymmetric division within the sphere. E) Second passage of 5BrdU-retaining mammospheres from (D) demonstrating that mammospheres contain cells that have retained nuclear label (5BrdU, green, arrow) even after dissociation and reseeding suggesting best explained by the presence of label retaining cells that have divided asymmetrically and retained originally labeled DNA strands. Nuclei stained with DAPI in D and E. Scale bars = 50μm in A and B, 20 μm in D and E.
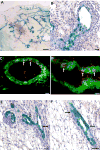
Figure 2
CDβgeo mammospheres reconstitute mammary glands. Mammospheres that formed from CDβgeo cells after 14 days in culture were transplanted into the cleared mammary fat pads of 3-week old female Nu/Nu mice. The resulting outgrowths were removed 8 weeks later, fixed, processed for Xgal staining, sectioned and counterstained with hematoxylin. A) Low power image of a mammary outgrowth demonstrating that Xgal positive (blue) CDβgeo cells have contributed progeny to the reconstitution of the gland. B) Higher power image of (A) showing CDβgeo cells in luminal positions in a subtending duct and developing acinar structure. C) Cross section of (A) demonstrating CDβgeo cell progeny (stained for β-gal, green) differentiate into ERα expressing cells (red). D) Cross section of (A) demonstrating CDβgeo cell progeny (stained for β-gal, green) differentiate into PR expressing cells (red). Mammospheres formed from a parous WAP-Cre/Rosa26R transgenic mouse in which the Cre recombinase has activated the Rosa26 reporter gene gave similar results. The lacZ+ cells are located in both E) ductal and acinar luminal positions and in F) myoepithelial positions (arrows). Scale bars = 1 mm in A, 20 μm in B, 10 μm in C and D, and 40 μm in E and F.
Figure 3
Mammosphere formation requires MAPK. A) Freshly isolated Balb/c mammary epithelial cells were seeded in mammosphere forming conditions with or without UO126. The numbers of mammospheres were counted visually at 4 days and 7 days after seeding. B) CDβgeo cells were seeded in mammosphere forming conditions with or without UO126. The numbers of mammospheres were counted visually at 4 days and 7 days after seeding. *p < 0.05 vs. untreated. Error bars indicate S.E.
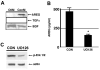
Figure 4
CDβgeo mammospheres release of amphiregulin is MAPK-dependent. A) Mammospheres formed from CDβgeo cells were grown for 7 days at which time the media was collected and immunoprecipitation performed on control media (CtlM) and conditioned media (ConM) using anti-AREG, anti-TGFα or anti-EGF antibodies. The immunoprecipitates were analyzed by Western blotting. B) CDβgeo mammospheres formed under normal conditions (CON) or in the presence of UO126 for 7 days at which time the conditioned media analyzed by ELISA for AREG (n=6, *p<0.05). C) Cell lysates from mammospheres were examined for p-Erk1/2 following treatment with the U0126 inhibitor or untreated (CON) after 7 days in culture.
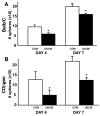
Figure 5
CDβgeo mammospheres formed in the presence of media alone (CON), anti-AREG (aAREG), AG1478 (AG) or anti-ADAM17 (aADAM). A) The number of mammospheres that formed was counted after 4 and 7 days in culture. B) After 4 and 7 days the conditioned media was collected and AREG was analyzed by ELISA (n=6, *p<0.05).
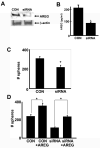
Figure 6
Transfection with siRNA directed towards AREG reduces AREG production and mammosphere formation. A) Western analysis following immunoprecipitation using anti-AREG of lysates from mammospheres that formed following AREG siRNA transfection (siRNA) or mock transfection (CON). B) Results of AREG ELISA demonstrating that siRNA decreases the levels of AREG in conditioned media. C) The number of mammospheres that form from CDβgeo cells after transfection is significantly reduced compared to control. D) The addition of exogenous AREG restores the CDβgeo mammosphere forming quality following AREG siRNA transfection. (*p < 0.05, scale bars = S.E.).
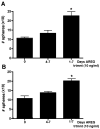
Figure 7
Addition of exogenous AREG rescues mammosphere capacity of AREG−/− mammary epithelial cells. A) AREG−/− mammary epithelial cells were seeded in mammosphere forming conditions (1000 cells/ml) with (right bar) or without (left bar) the addition of exogenous AREG. The middle bar demonstrates that when AREG is added after the fourth day in culture there is no change in mammosphere numbers when compared to the untreated (left bar) indicating that AREG is essential for mammosphere formation at the onset of formation. B) The mammospheres that formed in (A) were dissociated and reseeded as outlined in (A). After 7 days the number of mammospheres were counted. Left bar=untreated, middle bar mammospheres that received AREG at day 4 of culture, Right bar= mammospheres that received AREG from initial seeding. N=3, *p<0.01.
Figure 8
A subset of mammary progenitor cells responds to AREG inhibition. Examples of mammary outgrowths following transplantation with A) lobule-limited progenitor cells and C) duct-limited progenitor cells. Panels (B) and (D) are higher magnifications of (A) and (C) respectively. Transfection with siRNA directed towards AREG reduces mammosphere formation by duct-limited progenitor cells. Duct-limited CDβgeo cells (DUC-L), and CDβgeo lobule-limited cells (LOB-L) were untreated (CON), transfected with AREG siRNA (siRNA) or a scrambled control siRNA (SCR) and placed in mammosphere forming conditions. The numbers of mammospheres that formed were counted after E) 4 days and F) 7 days. N=6, *p<0.01 vs. CON.
Similar articles
- Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin.
Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Sternlicht MD, et al. Development. 2005 Sep;132(17):3923-33. doi: 10.1242/dev.01966. Epub 2005 Aug 3. Development. 2005. PMID: 16079154 Free PMC article. - The ADAM17-amphiregulin-EGFR axis in mammary development and cancer.
Sternlicht MD, Sunnarborg SW. Sternlicht MD, et al. J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):181-94. doi: 10.1007/s10911-008-9084-6. Epub 2008 May 10. J Mammary Gland Biol Neoplasia. 2008. PMID: 18470483 Free PMC article. Review. - Cyclin D1 determines estrogen signaling in the mammary gland in vivo.
Casimiro MC, Wang C, Li Z, Di Sante G, Willmart NE, Addya S, Chen L, Liu Y, Lisanti MP, Pestell RG. Casimiro MC, et al. Mol Endocrinol. 2013 Sep;27(9):1415-28. doi: 10.1210/me.2013-1065. Epub 2013 Jul 17. Mol Endocrinol. 2013. PMID: 23864650 Free PMC article. - In vivo reprogramming of non-mammary cells to an epithelial cell fate is independent of amphiregulin signaling.
George AL, Boulanger CA, Anderson LH, Cagnet S, Brisken C, Smith GH. George AL, et al. J Cell Sci. 2017 Jun 15;130(12):2018-2025. doi: 10.1242/jcs.200030. Epub 2017 Apr 28. J Cell Sci. 2017. PMID: 28455412 Free PMC article. - Amphiregulin: role in mammary gland development and breast cancer.
McBryan J, Howlin J, Napoletano S, Martin F. McBryan J, et al. J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):159-69. doi: 10.1007/s10911-008-9075-7. Epub 2008 Apr 9. J Mammary Gland Biol Neoplasia. 2008. PMID: 18398673 Review.
Cited by
- ErbB/EGF signaling and EMT in mammary development and breast cancer.
Hardy KM, Booth BW, Hendrix MJ, Salomon DS, Strizzi L. Hardy KM, et al. J Mammary Gland Biol Neoplasia. 2010 Jun;15(2):191-9. doi: 10.1007/s10911-010-9172-2. Epub 2010 Apr 6. J Mammary Gland Biol Neoplasia. 2010. PMID: 20369376 Free PMC article. Review. - Amphiregulin-dependent mucous cell metaplasia in a model of nonallergic lung injury.
Manzo ND, Foster WM, Stripp BR. Manzo ND, et al. Am J Respir Cell Mol Biol. 2012 Sep;47(3):349-57. doi: 10.1165/rcmb.2011-0257OC. Epub 2012 Apr 5. Am J Respir Cell Mol Biol. 2012. PMID: 22493011 Free PMC article. - Validation of an in vitro model of erbB2(+) cancer cell redirection.
Park JP, Blanding WM, Feltracco JA, Booth BW. Park JP, et al. In Vitro Cell Dev Biol Anim. 2015 Sep;51(8):776-86. doi: 10.1007/s11626-015-9889-8. Epub 2015 Apr 22. In Vitro Cell Dev Biol Anim. 2015. PMID: 25898824 - miRNA-34c-5p inhibits amphiregulin-induced ovarian cancer stemness and drug resistance via downregulation of the AREG-EGFR-ERK pathway.
Tung SL, Huang WC, Hsu FC, Yang ZP, Jang TH, Chang JW, Chuang CM, Lai CR, Wang LH. Tung SL, et al. Oncogenesis. 2017 May 1;6(5):e326. doi: 10.1038/oncsis.2017.25. Oncogenesis. 2017. PMID: 28459431 Free PMC article. - Protective effects of prepubertal genistein exposure on mammary tumorigenesis are dependent on BRCA1 expression.
de Assis S, Warri A, Benitez C, Helferich W, Hilakivi-Clarke L. de Assis S, et al. Cancer Prev Res (Phila). 2011 Sep;4(9):1436-48. doi: 10.1158/1940-6207.CAPR-10-0346. Epub 2011 Jun 16. Cancer Prev Res (Phila). 2011. PMID: 21680703 Free PMC article.
References
- Kenney NJ, Huang RP, Johnson GR, Wu JX, Okamura D, Matheny W, Kordon E, Gullick WJ, Plowman G, Smith GH, Salomon DS, Anderson ED. Detection and location of amphiregulin and Cripto-1 expression in the developing postnatal mouse mammary gland. Mol Reprod Dev. 1995;41:277–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials