SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease - PubMed (original) (raw)
SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease
Jeffrey B Schwimmer et al. Gastroenterology. 2010 Apr.
Abstract
Background & aims: The appropriate alanine aminotransferase (ALT) threshold value to use for diagnosis of chronic liver disease in children is unknown. We sought to develop gender-specific, biology-based, pediatric ALT thresholds.
Methods: The Screening ALT for Elevation in Today's Youth (SAFETY) study collected observational data from acute care children's hospitals, the National Health and Nutrition Examination Survey (NHANES, 1999-2006), overweight children with and without non-alcoholic fatty liver disease (NAFLD), and children with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infections. The study compared the sensitivity and specificity of ALT thresholds currently used by children's hospitals vs study-derived, gender-specific, biology-based, ALT thresholds for detecting children with NAFLD, HCV, or HBV.
Results: The median upper limit of ALT at children's hospitals was 53 U/L (range, 30-90 U/L). The 95th percentile levels for ALT in healthy weight, metabolically normal, liver disease-free, NHANES pediatric participants were 25.8 U/L (boys) and 22.1 U/L (girls). The concordance statistics of these NHANES-derived thresholds for liver disease detection were 0.85 (95% confidence interval [CI]: 0.74-0.96) in boys and 0.91 (95% CI: 0.83-0.99) in girls for NAFLD, 0.80 (95% CI: 0.70-0.91) in boys and 0.79 (95% CI: 0.69-0.89) in girls for HBV, and 0.86 (95% CI: 0.77-0.95) in boys and 0.84 (95% CI: 0.75-0.93) in girls for HCV. Using current children's hospitals ALT thresholds, the median sensitivity for detection of NAFLD, HBV, and HCV ranged from 32% to 48%; median specificity was 92% (boys) and 96% (girls). Using NHANES-derived thresholds, the sensitivities were 72% (boys) and 82% (girls); specificities were 79% (boys) and 85% (girls).
Conclusions: The upper limit of ALT used in children's hospitals varies widely and is set too high to reliably detect chronic liver disease. Biology-based thresholds provide higher sensitivity and only slightly less specificity. Clinical guidelines for use of screening ALT and exclusion criteria for clinical trials should be modified.
Keywords: clinical trials; hepatitis; nonalcoholic fatty liver disease; obesity; patient safety.
2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
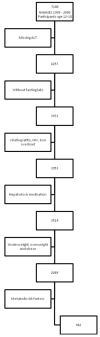
Figure 1. Inclusion and Exclusion for NHANES Reference Population
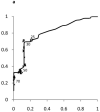
Figure 2. Receiver Operating Characteristic (ROC) curves for ALT in Boys and Girls
Shown in Figure 2 for boys and Figure 3 for girls are the sensitivity and [1-specificity] of ALT in detecting Hepatitis B Viral Infection (Figure 2A, 3A), Hepatitis C Viral Infection (Figure 2B, 3B), and Nonalcoholic Fatty Liver Disease (Figure 2C, 3C). Plotted along the ROC curves are the ALT thresholds currently utilized by children’s hospitals (marked with: x) and the biologically-derived ALT threshold (marked with: o). The numbers on the ROC curves represent the median and interquartile range of ALT thresholds currently used by children’s hospitals, as well as the biologically- derived ALT threshold.
Figure 2. Receiver Operating Characteristic (ROC) curves for ALT in Boys and Girls
Shown in Figure 2 for boys and Figure 3 for girls are the sensitivity and [1-specificity] of ALT in detecting Hepatitis B Viral Infection (Figure 2A, 3A), Hepatitis C Viral Infection (Figure 2B, 3B), and Nonalcoholic Fatty Liver Disease (Figure 2C, 3C). Plotted along the ROC curves are the ALT thresholds currently utilized by children’s hospitals (marked with: x) and the biologically-derived ALT threshold (marked with: o). The numbers on the ROC curves represent the median and interquartile range of ALT thresholds currently used by children’s hospitals, as well as the biologically- derived ALT threshold.
Figure 2. Receiver Operating Characteristic (ROC) curves for ALT in Boys and Girls
Shown in Figure 2 for boys and Figure 3 for girls are the sensitivity and [1-specificity] of ALT in detecting Hepatitis B Viral Infection (Figure 2A, 3A), Hepatitis C Viral Infection (Figure 2B, 3B), and Nonalcoholic Fatty Liver Disease (Figure 2C, 3C). Plotted along the ROC curves are the ALT thresholds currently utilized by children’s hospitals (marked with: x) and the biologically-derived ALT threshold (marked with: o). The numbers on the ROC curves represent the median and interquartile range of ALT thresholds currently used by children’s hospitals, as well as the biologically- derived ALT threshold.
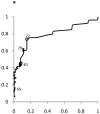
Figure 3. Receiver Operating Characteristic (ROC) curves for ALT in Boys and Girls
Shown in Figure 2 for boys and Figure 3 for girls are the sensitivity and [1-specificity] of ALT in detecting Hepatitis B Viral Infection (Figure 2A, 3A), Hepatitis C Viral Infection (Figure 2B, 3B), and Nonalcoholic Fatty Liver Disease (Figure 2C, 3C). Plotted along the ROC curves are the ALT thresholds currently utilized by children’s hospitals (marked with: x) and the biologically-derived ALT threshold (marked with: o). The numbers on the ROC curves represent the median and interquartile range of ALT thresholds currently used by children’s hospitals, as well as the biologically- derived ALT threshold.
Figure 3. Receiver Operating Characteristic (ROC) curves for ALT in Boys and Girls
Shown in Figure 2 for boys and Figure 3 for girls are the sensitivity and [1-specificity] of ALT in detecting Hepatitis B Viral Infection (Figure 2A, 3A), Hepatitis C Viral Infection (Figure 2B, 3B), and Nonalcoholic Fatty Liver Disease (Figure 2C, 3C). Plotted along the ROC curves are the ALT thresholds currently utilized by children’s hospitals (marked with: x) and the biologically-derived ALT threshold (marked with: o). The numbers on the ROC curves represent the median and interquartile range of ALT thresholds currently used by children’s hospitals, as well as the biologically- derived ALT threshold.
Figure 3. Receiver Operating Characteristic (ROC) curves for ALT in Boys and Girls
Shown in Figure 2 for boys and Figure 3 for girls are the sensitivity and [1-specificity] of ALT in detecting Hepatitis B Viral Infection (Figure 2A, 3A), Hepatitis C Viral Infection (Figure 2B, 3B), and Nonalcoholic Fatty Liver Disease (Figure 2C, 3C). Plotted along the ROC curves are the ALT thresholds currently utilized by children’s hospitals (marked with: x) and the biologically-derived ALT threshold (marked with: o). The numbers on the ROC curves represent the median and interquartile range of ALT thresholds currently used by children’s hospitals, as well as the biologically- derived ALT threshold.
Similar articles
- Normal serum alanine aminotransferase and non-alcoholic fatty liver disease among Korean adolescents: a cross-sectional study using data from KNHANES 2010-2015.
Kang Y, Park S, Kim S, Koh H. Kang Y, et al. BMC Pediatr. 2018 Jul 5;18(1):215. doi: 10.1186/s12887-018-1202-z. BMC Pediatr. 2018. PMID: 29976192 Free PMC article. - Upper limits of normal for serum alanine aminotransferase levels in Chinese Han population.
Zheng MH, Shi KQ, Fan YC, Liu WY, Lin XF, Li LF, Chen YP. Zheng MH, et al. PLoS One. 2012;7(9):e43736. doi: 10.1371/journal.pone.0043736. Epub 2012 Sep 4. PLoS One. 2012. PMID: 22962588 Free PMC article. - Updated upper limits of normal serum alanine aminotrasferase levels for screening metabolic dysfunction-associated fatty liver disease in obese children.
Lin YC, Chang PF, Ni YH. Lin YC, et al. J Formos Med Assoc. 2022 Dec;121(12):2548-2555. doi: 10.1016/j.jfma.2022.06.002. Epub 2022 Jun 21. J Formos Med Assoc. 2022. PMID: 35738972 - Does the hepatologist still need to rely on aminotransferases in clinical practice? A reappraisal of the role of a classic biomarker in the diagnosis and clinical management of chronic liver diseases.
Burra P, Cammà C, Invernizzi P, Marra F, Pompili M. Burra P, et al. Ann Hepatol. 2025 Jan-Jun;30(1):101900. doi: 10.1016/j.aohep.2025.101900. Epub 2025 Mar 13. Ann Hepatol. 2025. PMID: 40089150 Review. - Aminotransferase levels in clinical practice - what is normal?
Allam J, Rockey DC. Allam J, et al. Curr Opin Gastroenterol. 2025 Jul 1;41(4):260-264. doi: 10.1097/MOG.0000000000001094. Epub 2025 Apr 9. Curr Opin Gastroenterol. 2025. PMID: 40227983 Review.
Cited by
- Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010.
Welsh JA, Karpen S, Vos MB. Welsh JA, et al. J Pediatr. 2013 Mar;162(3):496-500.e1. doi: 10.1016/j.jpeds.2012.08.043. Epub 2012 Oct 17. J Pediatr. 2013. PMID: 23084707 Free PMC article. - Non-alcoholic fatty liver disease is associated with low bone mineral density in obese children.
Pardee PE, Dunn W, Schwimmer JB. Pardee PE, et al. Aliment Pharmacol Ther. 2012 Jan;35(2):248-54. doi: 10.1111/j.1365-2036.2011.04924.x. Epub 2011 Nov 24. Aliment Pharmacol Ther. 2012. PMID: 22111971 Free PMC article. - Nonalcoholic fatty liver disease in children with obesity- observations from one clinical centre in the Western Pomerania region.
Marcinkiewicz K, Horodnicka-Józwa A, Jackowski T, Strączek K, Biczysko-Mokosa A, Walczak M, Petriczko E. Marcinkiewicz K, et al. Front Endocrinol (Lausanne). 2022 Oct 31;13:992264. doi: 10.3389/fendo.2022.992264. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36387906 Free PMC article. - Persistent hypertransaminasemia in asymptomatic children: a stepwise approach.
Vajro P, Maddaluno S, Veropalumbo C. Vajro P, et al. World J Gastroenterol. 2013 May 14;19(18):2740-51. doi: 10.3748/wjg.v19.i18.2740. World J Gastroenterol. 2013. PMID: 23687411 Free PMC article. Review. - The phenotype of the pediatric patient with Cushing syndrome but without obesity.
Padilla C, Stratakis CA, Tatsi C. Padilla C, et al. Eur J Endocrinol. 2024 Sep 30;191(4):399-406. doi: 10.1093/ejendo/lvae114. Eur J Endocrinol. 2024. PMID: 39288098
References
- Jonas MM. Children with hepatitis C. Hepatology. 2002;36:S173–178. - PubMed
- Barlow S, the Expert Committee Expert Committee Recommendations on the Assessment, Prevention, and Treatment of Child and Adolescent Overweight and Obesity, Summary Report. Pediatrics. 2007;120:S164–S192. - PubMed
- Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R24 DK080506/DK/NIDDK NIH HHS/United States
- P60 MD000220/MD/NIMHD NIH HHS/United States
- P60 MD00220/MD/NIMHD NIH HHS/United States
- R21 DK071486/DK/NIDDK NIH HHS/United States
- T32 DK07202/DK/NIDDK NIH HHS/United States
- T32 DK007202/DK/NIDDK NIH HHS/United States
- R21 DK71486/DK/NIDDK NIH HHS/United States
- M01 RR000827/RR/NCRR NIH HHS/United States
- DK080506/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical