Positive regulation of interferon regulatory factor 3 activation by Herc5 via ISG15 modification - PubMed (original) (raw)
Positive regulation of interferon regulatory factor 3 activation by Herc5 via ISG15 modification
He-Xin Shi et al. Mol Cell Biol. 2010 May.
Abstract
Virus infection induces host antiviral responses, including induction of type I interferons. Transcription factor interferon regulatory factor 3 (IRF3) plays a pivotal role and is tightly regulated in this process. Here, we identify HERC5 (HECT domain and RLD 5) as a specific binding protein of IRF3 by immunoprecipitation. Ectopic expression or knockdown of HERC5 could, respectively, enhance or impair IRF3-mediated gene expression. Mechanistically, HERC5 catalyzes the conjugation of ubiquitin-like protein ISG15 onto IRF3 (Lys193, -360, and -366), thus attenuating the interaction between Pin1 and IRF3, resulting in sustained IRF3 activation. In contrast to results for wild-type IRF3, the mutant IRF3(K193,360,366R) interacts tightly with Pin1, is highly polyubiquitinated, and becomes less stable upon Sendai virus (SeV) infection. Consistently, host antiviral responses are obviously boosted or crippled in the presence or absence of HERC5, respectively. Collectively, this study characterizes HERC5 as a positive regulator of innate antiviral responses. It sustains IRF3 activation via a novel posttranslational modification, ISGylation.
Figures
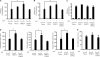
FIG. 1.
Identification of HERC5 in the IRF3 complex. (A) HEK293T cells transfected with Flag-IRF3 were mock infected or infected with SeV (MOI of 0.2) for 16 h, and then the cell lysates were subjected to immunoprecipitation with anti-Flag monoclonal antibody. The immunoprecipitates were resolved by SDS-PAGE followed by silver staining. The specific band indicated by the asterisk was excised for MS identification. (B) The same samples were immunoblotted (IB) with anti-HERC5 antibody. (C) HEK293T cells were cotransfected with the indicated constructs. Then, equal amounts of cell lysates were immunoprecipitated (IP) with anti-IgG or anti-Flag antibody. The immunoprecipitates were immunoblotted with the indicated antibodies. (D) HEK293T cells were cotransfected with the indicated constructs. Then, equal amounts of cell lysates were immunoprecipitated with anti-IgG or anti-HA antibody. The immunoprecipitates were immunoblotted with the indicated antibodies. (E) After mock or poly(I-C) (transfected, 2 μg/ml) stimulation, lysates from HEK293 cells were immunoprecipitated with anti-IRF3 antibody or anti-IgG and then immunoblotted with anti-HERC5 antibody. (F) Schematic diagram of IRF3 and its truncation mutants (right) (DBD, DNA-binding domain; IAD, IRF association domain; FL, full length). Flag-IRF3 mutants were individually transfected into HEK293T cells along with HA-HERC5. The cell lysates were immunoprecipitated with anti-IgG or anti-Flag antibody and immunoblotted with the indicated antibodies (left).
FIG. 2.
HERC5 synergizes IRF3 activation. (A to C) Equal amounts of the indicated plasmids (50 ng) were transfected into HEK293 cells along with the IFN-β (A), PRDIII-I (B), or NF-κB (C) reporter plasmid. Twenty-four hours after transfection, cells were infected with or without SeV (MOI of 0.2). The luciferase (luc) assay was performed 12 h postinfection. A pTK-Renilla reporter was used to normalize data. (D and E) Induction of IFN-β, ISG54, RANTES (D), and IL-8 (E) mRNA by SeV infection (MOI of 0.2) in the presence of a control and the indicated plasmids (50 ng) was measured by Q-PCR. Data are presented as means ± standard deviations (SD) (n = 3 replicates). *, P < 0.05; **, P < 0.01.
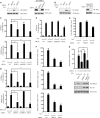
FIG. 3.
Knockdown of HERC5 attenuates IRF3 activation. (A) HEK293 cells were transfected with NC or HERC5 siRNA and then mock infected or infected with SeV. Cell lysates were immunoblotted with anti-HERC5 antibody (left). HEK293 cells were transfected with HA-HERC5 and then treated with NC, HERC5, or mtHERC5 siRNA. Cell lysates were immunoblotted with anti-HA antibody (right). (B) HEK293 cells were transfected with NC or ISG15 siRNA and then mock infected or infected with SeV (MOI of 0.2). Cell lysates were immunoblotted with anti-ISG15 antibody (left). HEK293 cells were transfected with Flag-ISG15 and then treated with NC or ISG15 siRNA. Cell lysates were immunoblotted with anti-Flag antibody (right). (C) The indicated siRNAs were transfected into HEK293 cells together with the IFN-β or PRDIII-I reporter plasmid. Forty-eight hours after transfection, cells were infected with SeV (MOI of 0.2) or not infected. The luciferase assay was performed 12 h postinfection. A pTK-Renilla reporter was used to normalize the data. (D) The indicated siRNAs were transfected into HEK293 cells together with the IFN-β or PRDIII-I reporter plasmid, and 24 h later, cells were transfected with IRF3 5D (S396, S398, S402, T404, and S405 all mutated to D). (E) The indicated siRNAs were transfected into HEK293 cells together with the NF-κB luciferase or pTK-Renilla reporter. Forty-eight hours later, cells were infected with SeV (MOI of 0.2). (F and G) Induction of IFN-β, ISG54, RANTES (F), and IL-8 (G) mRNA by SeV infection in the presence of a control and the indicated siRNAs was measured by Q-PCR. (H) HEK293 cells were transfected with the indicated siRNAs for 24 h. Then, siRNA-resistant HA-rHERC5 (50 ng) or HA-rHERC5 C994A (50 ng) was transfected into the knockdown cells. After SeV infection, induction of IFN-β mRNA was measured by Q-PCR. Data are presented as means ± SD (n = 3 replicates). NC, nonspecific control; mtHERC5 siRNA, a mutant form of HERC5 siRNA without silencing activity. *, P < 0.05; **, P < 0.01.
FIG. 4.
HERC5 catalyzes the conjugation of ISG15 onto IRF3. (A) Recombinant ISG15 and ubiquitin were resolved by SDS-PAGE and probed with anti-ISG15 (top) or anti-Ub (bottom). (B) HEK293 cells were mock infected or infected with increasing doses of SeV. Cell lysates were subjected to immunoprecipitation and then immunoblotted with the indicated antibodies. The positions of ISGylated IRF3 are shown by braces at right. (C) HEK293 cells were transfected with the indicated siRNAs. After SeV infection, cell lysates were subjected to immunoprecipitation and immunoblotted with the indicated antibodies. (D and E) HEK293T cells were transfected with the indicated plasmids. Twenty-four hours after transfection, cell lysates were subjected to immunoprecipitation (D) or Ni-NTA pulldown (PD) (E) and then immunoblotted with the indicated antibodies. (F) HEK293T cells expressing the indicated plasmids were subjected to Ni-NTA pulldown and then immunoblotted with the indicated antibodies. S2A, IRF3(S385,386A).
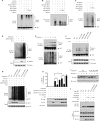
FIG. 5.
HERC5 enhances IRF3 stability. (A) HEK293 cells were transfected with the indicated siRNAs and then infected with SeV (MOI of 1.0) for the indicated times (h.p.i., hours postinfection). Cell lysates were immunoblotted with anti-IRF3 antibody. (B) HEK293 cells were transfected with HA-HERC5 (200 ng) or control. After infection with SeV (MOI of 1.0) for the indicated times, cell lysates were immunoblotted with anti-IRF3 antibody. Representative results are shown, and the amounts of IRF3 were measured densitometrically (right). Each datum point represents single-well samples.
FIG. 6.
HERC5 inhibits IRF3 ubiquitination. (A and B) HEK293T cells were transfected with the indicated plasmids and infected with SeV (MOI of 0.5). Cell lysates were subjected to immunoprecipitation (A) or Ni-NTA pulldown (B) and then immunoblotted with the indicated antibodies. (C) HEK293T cells were transfected with the indicated plasmids and infected with SeV (MOI of 0.5). Cell lysates were immunoprecipitated with rabbit anti-IRF3 antibody and then immunoblotted with mouse anti-Ub antibody. (D) HEK293 cells were treated with the indicated siRNAs. After mock infection or SeV infection, cell lysates were immunoprecipitated with rabbit anti-IRF3 antibody and then immunoblotted with mouse anti-Ub antibody. (E) HEK293 cells were infected with SeV (MOI of 0.5) for the indicated times. Cell lysates were immunoprecipitated with anti-ISG15 antibody and then immunoblotted with anti-IRF3 antibody (top) or immunoprecipitated with anti-IRF3 antibody and then immunoblotted with anti-Ub antibody (bottom). (F) HEK293 cells were transfected with Flag-IRF3 lysine mutants and then infected with SeV (MOI of 0.5). Cell lysates were subjected to immunoprecipitation with anti-Flag antibody and immunoblotted with anti-ISG15 antibody. (G) HEK293 cells were transfected with NC or siHERC5 for 24 h and then transfected with the indicated plasmids before infection with SeV (MOI of 0.5). Cell lysates were subjected to immunoprecipitation and then immunoblotted with the indicated antibodies. (H) The indicated plasmids (Flag-IRF3 lysine mutants at 500 ng per well and HA-HERC5 at 400 ng per well) were transfected into IRF3−/− MEF along with the IFN-β reporter plasmid. Twenty-four hours after transfection, cells were mock infected or infected with SeV (MOI of 0.8). The luciferase assay was performed 18 h postinfection. A pTK-Renilla reporter was used to normalize the data. The data are presented as means ± SD (n = 3). *, P < 0.05; **, P < 0.01. (I) IRF3−/− MEF were transfected with 200 ng of Flag-tagged WT IRF3 or Flag-IRF3(K193,360,366R) and then infected with SeV (MOI of 2) for the indicated times. Cell lysates were immunoblotted with anti-Flag antibody. (J) HEK293 cells were transfected with the indicated plasmids and then infected with SeV for 4 h. Cell lysates were subjected to GST pulldown and then immunoblotted with the indicated antibodies. The relative amounts of IRF3 were quantified by densitometry and normalized with respect to that shown in lane 2.
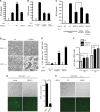
FIG. 7.
HERC5 modulates IRF3-mediated antiviral responses. (A and B) HEK293 cells were transfected with the indicated plasmids (50 ng) (A) or siRNAs (B). Six hours after SeV infection, IFN-β production was determined by ELISA. (C) The indicated siRNAs were transfected into HEK293 cells in the presence or absence of siRNA-resistant HERC5. Six hours after SeV infection, IFN-β production was determined by ELISA. (D) HEK293 cells were transfected with the indicated siRNAs. Twenty-four hours posttransfection, the cells were infected with NDV for an additional 20 h. The cytopathic effects (CPE) were observed by differential interference contrast DIC microscopy. Original magnification, ×100. (E) HEK293 cells were transfected with the indicated siRNAs. Twenty-four hours posttransfection, the cells were infected with SeV for an additional 10 h. Equal amounts of the culture supernatants (approximately 100 μl) were applied to fresh HEK293 cells for 6 h, followed by VSV infection. The titers of VSV were determined by a standard plaque assay. (F) HEK293 cells were transfected with the indicated siRNAs. Twenty-four hours posttransfection, the cells were infected with SeV for different times. Equal amounts of the conditioned medium with anti-IFN-α/β (20 μg/ml) were applied to Vero cells. A plaque assay for SeV was carried out using Vero cells as described previously. (G and H) NDV-GFP replication in HEK293 cells transfected with exogenous HERC5 (G) or HERC5 siRNA (H) was visualized by fluorescence microscopy. Original magnification, ×100. GFP-positive cells were quantified (G, right). Data are presented as means ± SD (n = 3 replicates). ND, not detected. *, P < 0.05; **, P < 0.01.
Similar articles
- Kaposi's Sarcoma-Associated Herpesvirus Viral Interferon Regulatory Factor 1 Interacts with a Member of the Interferon-Stimulated Gene 15 Pathway.
Jacobs SR, Stopford CM, West JA, Bennett CL, Giffin L, Damania B. Jacobs SR, et al. J Virol. 2015 Nov;89(22):11572-83. doi: 10.1128/JVI.01482-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355087 Free PMC article. - ISGylation of the SARS-CoV-2 N protein by HERC5 impedes N oligomerization and thereby viral RNA synthesis.
Zhu J, Liu G, Sayyad Z, Goins CM, Stauffer SR, Gack MU. Zhu J, et al. J Virol. 2024 Sep 17;98(9):e0086924. doi: 10.1128/jvi.00869-24. Epub 2024 Aug 28. J Virol. 2024. PMID: 39194248 - HERC5 is an IFN-induced HECT-type E3 protein ligase that mediates type I IFN-induced ISGylation of protein targets.
Wong JJ, Pung YF, Sze NS, Chin KC. Wong JJ, et al. Proc Natl Acad Sci U S A. 2006 Jul 11;103(28):10735-40. doi: 10.1073/pnas.0600397103. Epub 2006 Jun 30. Proc Natl Acad Sci U S A. 2006. PMID: 16815975 Free PMC article. - HERC5 and the ISGylation Pathway: Critical Modulators of the Antiviral Immune Response.
Mathieu NA, Paparisto E, Barr SD, Spratt DE. Mathieu NA, et al. Viruses. 2021 Jun 9;13(6):1102. doi: 10.3390/v13061102. Viruses. 2021. PMID: 34207696 Free PMC article. Review. - The antiviral activities of ISG15.
Morales DJ, Lenschow DJ. Morales DJ, et al. J Mol Biol. 2013 Dec 13;425(24):4995-5008. doi: 10.1016/j.jmb.2013.09.041. Epub 2013 Oct 3. J Mol Biol. 2013. PMID: 24095857 Free PMC article. Review.
Cited by
- Emerging roles of interferon-stimulated genes in the innate immune response to hepatitis C virus infection.
Wong MT, Chen SS. Wong MT, et al. Cell Mol Immunol. 2016 Jan;13(1):11-35. doi: 10.1038/cmi.2014.127. Epub 2014 Dec 29. Cell Mol Immunol. 2016. PMID: 25544499 Free PMC article. Review. - ISG15-dependent activation of the sensor MDA5 is antagonized by the SARS-CoV-2 papain-like protease to evade host innate immunity.
Liu G, Lee JH, Parker ZM, Acharya D, Chiang JJ, van Gent M, Riedl W, Davis-Gardner ME, Wies E, Chiang C, Gack MU. Liu G, et al. Nat Microbiol. 2021 Apr;6(4):467-478. doi: 10.1038/s41564-021-00884-1. Epub 2021 Mar 16. Nat Microbiol. 2021. PMID: 33727702 Free PMC article. - The E3 ubiquitin ligase ARIH1 promotes antiviral immunity and autoimmunity by inducing mono-ISGylation and oligomerization of cGAS.
Xiong TC, Wei MC, Li FX, Shi M, Gan H, Tang Z, Dong HP, Liuyu T, Gao P, Zhong B, Zhang ZD, Lin D. Xiong TC, et al. Nat Commun. 2022 Oct 10;13(1):5973. doi: 10.1038/s41467-022-33671-5. Nat Commun. 2022. PMID: 36217001 Free PMC article. - ISG15 deficiency restricts HIV-1 infection.
Jurczyszak D, Manganaro L, Buta S, Gruber C, Martin-Fernandez M, Taft J, Patel RS, Cipolla M, Alshammary H, Mulder LCF, Sachidanandam R, Bogunovic D, Simon V. Jurczyszak D, et al. PLoS Pathog. 2022 Mar 25;18(3):e1010405. doi: 10.1371/journal.ppat.1010405. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35333911 Free PMC article. - Coronaviral PLpro proteases and the immunomodulatory roles of conjugated versus free Interferon Stimulated Gene product-15 (ISG15).
Gold IM, Reis N, Glaser F, Glickman MH. Gold IM, et al. Semin Cell Dev Biol. 2022 Dec;132:16-26. doi: 10.1016/j.semcdb.2022.06.005. Epub 2022 Jun 25. Semin Cell Dev Biol. 2022. PMID: 35764457 Free PMC article. Review.
References
- Bibeau-Poirier, A., S. P. Gravel, J. F. Clement, S. Rolland, G. Rodier, P. Coulombe, J. Hiscott, N. Grandvaux, S. Meloche, and M. J. Servant. 2006. Involvement of the IkappaB kinase (IKK)-related kinases tank-binding kinase 1/IKKi and cullin-based ubiquitin ligases in IFN regulatory factor-3 degradation. J. Immunol. 177:5059-5067. - PubMed
- Cruz, C., F. Ventura, R. Bartrons, and J. L. Rosa. 2001. HERC3 binding to and regulation by ubiquitin. FEBS Lett. 488:74-80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous