Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation - PubMed (original) (raw)
Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation
Claire Barbier de La Serre et al. Am J Physiol Gastrointest Liver Physiol. 2010 Aug.
Abstract
Consumption of diets high in fat and calories leads to hyperphagia and obesity, which is associated with chronic "low-grade" systemic inflammation. Ingestion of a high-fat diet alters the gut microbiota, pointing to a possible role in the development of obesity. The present study used Sprague-Dawley rats that, when fed a high-fat diet, exhibit either an obesity-prone (DIO-P) or obesity-resistant (DIO-R) phenotype, to determine whether changes in gut epithelial function and microbiota are diet or obese associated. Food intake and body weight were monitored daily in rats maintained on either low- or high-fat diets. After 8 or 12 wk, tissue was removed to determine adiposity and gut epithelial function and to analyze the gut microbiota using PCR. DIO-P but not DIO-R rats exhibit an increase in toll-like receptor (TLR4) activation associated with ileal inflammation and a decrease in intestinal alkaline phosphatase, a luminal enzyme that detoxifies lipopolysaccharide (LPS). Intestinal permeability and plasma LPS were increased together with phosphorylation of myosin light chain and localization of occludin in the cytoplasm of epithelial cells. Measurement of bacterial 16S rRNA showed a decrease in total bacterial density and an increase in the relative proportion of Bacteroidales and Clostridiales orders in high-fat-fed rats regardless of phenotype; an increase in Enterobacteriales was seen in the microbiota of DIO-P rats only. Consumption of a high-fat diet induces changes in the gut microbiota, but it is the development of inflammation that is associated with the appearance of hyperphagia and an obese phenotype.
Figures
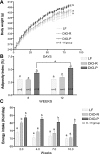
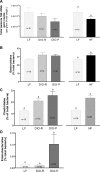
Fig. 1.
Effect of consumption of a high-fat (HF) diet on body weight, adiposity, and food intake in low-fat (LF) and HF-fed animals after 8 or 12 wk on respective diets A: there was a significant increase in body weight in diet-induced obesity-prone (DIO-P) rats compared with diet-induced obesity-resistant (DIO-R) or LF animals (DIO-P vs. LF, P < 0.001, DIO-P vs. DIO-R, P < 0.01). B: adiposity index was calculated as the sum of the fat pads expressed as a percentage of total body weight. After 8 or 12 wk on the diets, DIO-P rats showed a significantly higher adiposity than LF and DIO-R rats (DIO-P vs. LF or DIO-R, P < 0.01 at 8 wk, P < 0.001 at 12 wk). There was a significant increase in adiposity after 12 wk compared with 8 wk (*P < 0.05) in DIO-P rats. C: starting at week 4, the DIO-P group had a significantly higher energy intake than LF and DIO-R animals (week 4: DIO-P vs. LF, P < 0.001, DIO-P vs. DIO-R, P < 0.01; week 7: DIO-P vs. LF or DIO-R, P < 0.05; week 10: DIO-P vs. LF or DIO-R, P < 0.001). Data are means ± SE; n, no. of rats. Different letters denote significant differences between groups.
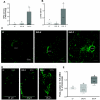
Fig. 2.
A: myeloperoxidase (MPO) activity in the ileum in LF, DIO-R, and DIO-P rats after 12 wk on respective diets. There was a significantly higher level of MPO activity in DIO-P rats compared with LF or DIO-R animals (DIO-P vs. LF, P < 0.01, DIO-P vs. DIO-R, P < 0.05). B: plasma levels of lipopolysaccharide (LPS) were increased in DIO-P but not DIO-R rats after 12 wk on a HF diet (DIO-P vs. LF or DIO-R, P < 0.05). C and D: photomicrographs of toll-like receptor (TLR)4/MD2 complex immunoreactivity in transverse sections of ileal crypts from LF, DIO-R, and DIO-P rats after 12 wk on respective diets. E: quantification of TLR4/MD2 immunoreactivity expressed as a percentage of positive pixels from 4–6 images/rat; n = 4/group. There was more immunoreactivity in DIO-P than LF and DIO-R tissue (DIO-P vs. LF or DIO-R, P < 0.05). Different letters denote significant differences between groups.
Fig. 3.
Duodenal intestinal alkaline phosphatase (IAP) activity in LF, DIO-R, and DIO-P rats after 8 wk on respective diets. DIO-P rats had significantly lower IAP activity than LF and DIO-R animals (DIO-P vs. LF, P < 005, DIO-P vs. DIO-R, P < 001). Different letters denote significant differences between groups.
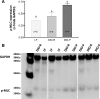
Fig. 4.
Phosphorylated myosin light chain (p-MLC) expression in the ileum in LF, DIO-R, and DIO-P rats after 12 wk on respective diets. A: p-MLC expression was measured by Western blot and quantified as a proportion of control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. The DIO-P group showed an increase in p-MLC expression compared with LF and DIO-R rats (DIO-P vs. LF, P < 0.01, DIO-P vs. DIO-R, P < 0.05). Different letters denote significant differences between groups. B: blot showing GAPDH and p-MLC expression in LF, DIO-R, and DIO-P rats after 12 wk on respective diets.
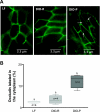
Fig. 5.
Immunolocalization of occludin in ileal sections in LF, DIO-R, and DIO-P rats after 12 wk on respective diets. A: photomicrographs of ileal mucosa in LF, DIO-R, and DIO-P rats showing immunolocalization of occluding to tight junctions; in ileum from DIO-P rats, immunoreactive occludin can be seen within the cytoplasm of the enterocytes. B: occludin localization was quantified by determination of %positive pixels in the cytoplasm. DIO-P rats showed an increase in occludin localization to the cytoplasm compared with LF and DIO-R (DIO-P vs. LF or DIO-R, 3–6 cells/image, 5 images/rat, n = 4/group; P < 0.001). Different letters denote significant differences between groups.
Fig. 6.
Measurement of gut permeability by appearance of FITC-labeled dextran in plasma in LF, DIO-R, and DIO-P rats after 10 wk on respective diets. DIO-P rats exhibited a significant increase in gut permeability compared with LF and DIO-R animals (DIO-P vs. LF or DIO-R, P < 0.001). DIO-R rats also exhibited a significant increase in gut permeability compared with the LF control group (DIO-R vs. LF, P < 0.001). Different letters denote significant differences between groups.
Fig. 7.
Quantification of total bacterial-universal 16S rRNA gene copies and proportion of bacterial order in cecal samples from LF, DIO-R, and DIO-P after 8 wk on respective diets. A: no significant difference in total bacterial 16S copies in LF, DIO-R, and DIO-P (LF vs. DIO-R, P = 0.3, LF vs. DIO-P, P = 0.1, DIO-R vs. DIO-P, P = 0.5); however, there was a significant decrease in total bacterial 16S copies in HF- vs. LF-fed rats (P < 0.05). B: Bacteroidales order as a relative percentage of total bacteria in cecal samples from rats after 8 wk on respective diets; there was no significant difference between LF, DIO-R, and DIO-P but a significant decrease in HF- vs. LF-fed rats (P < 0.01). C: Clostridiales order as a percentage of total bacteria in cecal samples from LF, DIO-R, and DIO-P after 8 wk on respective diets. DIO-R and DIO-P animals showed a significant increase in Clostridiales order compared with LF rats (DIO-R vs. LF, P < 0.05, DIO-P vs. LF, P < 0.01). There were no differences between DIO-R and DIO-P (P = 0.3), and there was a significant increase in Clostridiales order in HF vs. LF. rats (P < 0.001). D: Enterobacteriales order as a percentage of total bacteria in cecal samples from LF, DIO-R, and DIO-P after 8 wk on respective diets showing a significant increase in HF-fed DIO-P rats only (DIO-P vs. LF or DIO-R, P < 0.05).
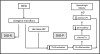
Fig. 8.
Proposed model by which ingestion of HF diets leads to hyperphagia and obesity. It is proposed that ingestion of a HF diet leads to changes in gut microbiota, which, possibly with a decrease in IAP, leads to an increase in luminal LPS and TLR4 activation in epithelial cells. The resulting gastrointestinal (GI) inflammation results in altered intestinal barrier function and an increase in passage of LPS from the lumen to the lamina propria. The precise mechanism by which this low but chronic increase in plasma LPS leads to altered regulation of food intake, hyperphagia, and obesity is not clear. TJs, tight junctions.
Similar articles
- Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity.
Sen T, Cawthon CR, Ihde BT, Hajnal A, DiLorenzo PM, de La Serre CB, Czaja K. Sen T, et al. Physiol Behav. 2017 May 1;173:305-317. doi: 10.1016/j.physbeh.2017.02.027. Epub 2017 Feb 27. Physiol Behav. 2017. PMID: 28249783 Free PMC article. - Apple-Derived Pectin Modulates Gut Microbiota, Improves Gut Barrier Function, and Attenuates Metabolic Endotoxemia in Rats with Diet-Induced Obesity.
Jiang T, Gao X, Wu C, Tian F, Lei Q, Bi J, Xie B, Wang HY, Chen S, Wang X. Jiang T, et al. Nutrients. 2016 Feb 29;8(3):126. doi: 10.3390/nu8030126. Nutrients. 2016. PMID: 26938554 Free PMC article. - Alterations to the microbiota-colon-brain axis in high-fat-diet-induced obese mice compared to diet-resistant mice.
Zhang P, Yu Y, Qin Y, Zhou Y, Tang R, Wang Q, Li X, Wang H, Weston-Green K, Huang XF, Zheng K. Zhang P, et al. J Nutr Biochem. 2019 Mar;65:54-65. doi: 10.1016/j.jnutbio.2018.08.016. Epub 2018 Sep 1. J Nutr Biochem. 2019. PMID: 30623851 - Translational research into gut microbiota: new horizons in obesity treatment.
Tsukumo DM, Carvalho BM, Carvalho-Filho MA, Saad MJ. Tsukumo DM, et al. Arq Bras Endocrinol Metabol. 2009 Mar;53(2):139-44. doi: 10.1590/s0004-27302009000200004. Arq Bras Endocrinol Metabol. 2009. PMID: 19466205 Retracted. Review. - Impact of dietary fat on gut microbiota and low-grade systemic inflammation: mechanisms and clinical implications on obesity.
Cândido FG, Valente FX, Grześkowiak ŁM, Moreira APB, Rocha DMUP, Alfenas RCG. Cândido FG, et al. Int J Food Sci Nutr. 2018 Mar;69(2):125-143. doi: 10.1080/09637486.2017.1343286. Epub 2017 Jul 4. Int J Food Sci Nutr. 2018. PMID: 28675945 Review.
Cited by
- Physiopathological Roles of White Adiposity and Gut Functions in Neuroinflammation.
Spinedi E, Docena GH. Spinedi E, et al. Int J Mol Sci. 2024 Oct 31;25(21):11741. doi: 10.3390/ijms252111741. Int J Mol Sci. 2024. PMID: 39519291 Free PMC article. Review. - Transfer of microbiota from lean donors in combination with prebiotics prevents excessive weight gain and improves gut-brain vagal signaling in obese rats.
Minaya DM, Kim JS, Kirkland R, Allen J, Cullinan S, Maclang N, de Lartigue G, de La Serre C. Minaya DM, et al. Gut Microbes. 2024 Jan-Dec;16(1):2421581. doi: 10.1080/19490976.2024.2421581. Epub 2024 Nov 1. Gut Microbes. 2024. PMID: 39485288 Free PMC article. - Yoyo Dieting, Post-Obesity Weight Loss, and Their Relationship with Gut Health.
Phuong-Nguyen K, McGee SL, Aston-Mourney K, Mcneill BA, Mahmood MQ, Rivera LR. Phuong-Nguyen K, et al. Nutrients. 2024 Sep 19;16(18):3170. doi: 10.3390/nu16183170. Nutrients. 2024. PMID: 39339770 Free PMC article. Review. - Sex-dependent effects of a high fat diet on metabolic disorders, intestinal barrier function and gut microbiota in mouse.
Lefebvre C, Tiffay A, Breemeersch CE, Dreux V, Bôle-Feysot C, Guérin C, Breton J, Maximin E, Monnoye M, Déchelotte P, Douard V, Goichon A, Coëffier M. Lefebvre C, et al. Sci Rep. 2024 Aug 27;14(1):19835. doi: 10.1038/s41598-024-70931-4. Sci Rep. 2024. PMID: 39191839 Free PMC article. - Soybean oil-based HFD induces gut dysbiosis that leads to steatosis, hepatic inflammation and insulin resistance in mice.
Jacob T, Sindhu S, Hasan A, Malik MZ, Arefanian H, Al-Rashed F, Nizam R, Kochumon S, Thomas R, Bahman F, Shenouda S, Wilson A, Akther N, Al-Roub A, Abukhalaf N, Albeloushi S, Abu-Farha M, Al Madhoun A, Alzaid F, Thanaraj TA, Koistinen HA, Tuomilehto J, Al-Mulla F, Ahmad R. Jacob T, et al. Front Microbiol. 2024 Aug 6;15:1407258. doi: 10.3389/fmicb.2024.1407258. eCollection 2024. Front Microbiol. 2024. PMID: 39165573 Free PMC article.
References
- Alpers DH, Zhang Y, Ahnen DJ. Synthesis and parallel secretion of rat intestinal alkaline phosphatase and a surfactant-like particle protein. Am J Physiol Endocrinol Metab 268: E1205–E1214, 1995 - PubMed
- Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferrieres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 87: 1219–1223, 2008 - PubMed
- Backhed F. Changes in intestinal microflora in obesity: cause or consequence? J Pediatr Gastroenterol Nutr 48, Suppl 2: S56–S57, 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous