Effect of preventive (beta blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: randomised controlled trial - PubMed (original) (raw)
Randomized Controlled Trial
Effect of preventive (beta blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: randomised controlled trial
Kenneth A Holroyd et al. BMJ. 2010.
Abstract
Objective: To determine if the addition of preventive drug treatment (β blocker), brief behavioural migraine management, or their combination improves the outcome of optimised acute treatment in the management of frequent migraine.
Design: Randomised placebo controlled trial over 16 months from July 2001 to November 2005.
Setting: Two outpatient sites in Ohio, USA.
Participants: 232 adults (mean age 38 years; 79% female) with diagnosis of migraine with or without aura according to International Headache Society classification of headache disorders criteria, who recorded at least three migraines with disability per 30 days (mean 5.5 migraines/30 days), during an optimised run-in of acute treatment.
Interventions: Addition of one of four preventive treatments to optimised acute treatment: β blocker (n=53), matched placebo (n=55), behavioural migraine management plus placebo (n=55), or behavioural migraine management plus β blocker (n=69).
Main outcome measure: The primary outcome was change in migraines/30 days; secondary outcomes included change in migraine days/30 days and change in migraine specific quality of life scores.
Results: Mixed model analysis showed statistically significant (P≤0.05) differences in outcomes among the four added treatments for both the primary outcome (migraines/30 days) and the two secondary outcomes (change in migraine days/30 days and change in migraine specific quality of life scores). The addition of combined β blocker and behavioural migraine management (-3.3 migraines/30 days, 95% confidence interval -3.2 to -3.5), but not the addition of β blocker alone (-2.1 migraines/30 days, -1.9 to -2.2) or behavioural migraine management alone (-2.2 migraines migraines/30 days, -2.0 to -2.4), improved outcomes compared with optimised acute treatment alone (-2.1 migraines/30 days, -1.9 to -2.2). For a clinically significant (≥50% reduction) in migraines/30 days, the number needed to treat for optimised acute treatment plus combined β blocker and behavioural migraine management was 3.1 compared with optimised acute treatment alone, 2.6 compared with optimised acute treatment plus β blocker, and 3.1 compared with optimised acute treatment plus behavioural migraine management. Results were consistent for the two secondary outcomes, and at both month 10 (the primary endpoint) and month 16.
Conclusion: The addition of combined β blocker plus behavioural migraine management, but not the addition of β blocker alone or behavioural migraine management alone, improved outcomes of optimised acute treatment. Combined β blocker treatment and behavioural migraine management may improve outcomes in the treatment of frequent migraine.
Trial registration: Clinical trials NCT00910689.
Conflict of interest statement
Competing interests: KAH has consulted for ENDO Pharmaceuticals and for Takeda Pharmaceuticals North America and received an investigator initiated grant from ENDO Pharmaceuticals. He has also received support from the National Institutes of Health (NINDS; NS32375). CKC has received research funding and materials from GlaxoSmithKline Pharmaceuticals (GSK) and Merck and participates in industry sponsored research involving GSK, Merck, UCB Pharma, and Allergan. FJO’D has received research funding and materials from GSK and Merck; receives educational funding from GSK, Merck, and Allergan; participates in industry sponsored research involving GSK, Merck, UCB Pharma, and Allergan; and has consulted for and received honorariums from GSK. GEC owns stock in Johnson and Johnson, Novartis, and Wyeth Pharmaceuticals.
Figures
Fig 1 Flow of participants in trial. *Reported that other time demands prevented completion of trial tasks (such as completing study appointments). †Side effects attributed to triptan (T) or β blocker (B). ‡Discontinued from trial for protocol violation (for example, failed to take preventive drug). §Decided to become or became pregnant
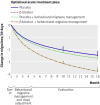
Fig 2 Mean change in number of migraines per 30 days from run-in of optimised acute treatment estimated for each of four treatments. Vertical lines at months 10 and 16 indicate primary end point and longer term assessment point
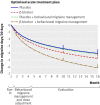
Fig 3 Mean change in number of migraine days per 30 days from run-in of optimised acute treatment estimated for each of four treatments. Vertical lines at months 10 and 16 indicate primary end point and longer term assessment point
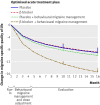
Fig 4 Mean change in migraine specific quality of life scores from run-in of optimised acute treatment estimated for each of four treatments. Vertical lines at months 10 and 16 indicate primary end point and longer term assessment point
Comment in
- Prevention of migraine.
Dodick DW. Dodick DW. BMJ. 2010 Sep 29;341:c5229. doi: 10.1136/bmj.c5229. BMJ. 2010. PMID: 20880899 No abstract available.
Similar articles
- Efficacy and safety of exogenous ketone bodies for preventive treatment of migraine: A study protocol for a single-centred, randomised, placebo-controlled, double-blind crossover trial.
Gross E, Putananickal N, Orsini AL, Schmidt S, Vogt DR, Cichon S, Sandor P, Fischer D. Gross E, et al. Trials. 2019 Jan 17;20(1):61. doi: 10.1186/s13063-018-3120-7. Trials. 2019. PMID: 30654835 Free PMC article. - SumaRT/Nap vs naproxen sodium in treatment and disease modification of migraine: a pilot study.
Cady R, O'Carroll P, Dexter K, Freitag F, Shade CL. Cady R, et al. Headache. 2014 Jan;54(1):67-79. doi: 10.1111/head.12211. Epub 2013 Sep 10. Headache. 2014. PMID: 24021029 Clinical Trial. - Evaluation of Galcanezumab for the Prevention of Episodic Migraine: The EVOLVE-1 Randomized Clinical Trial.
Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Stauffer VL, et al. JAMA Neurol. 2018 Sep 1;75(9):1080-1088. doi: 10.1001/jamaneurol.2018.1212. JAMA Neurol. 2018. PMID: 29813147 Free PMC article. Clinical Trial. - Beta-blockers for the prevention of headache in adults, a systematic review and meta-analysis.
Jackson JL, Kuriyama A, Kuwatsuka Y, Nickoloff S, Storch D, Jackson W, Zhang ZJ, Hayashino Y. Jackson JL, et al. PLoS One. 2019 Mar 20;14(3):e0212785. doi: 10.1371/journal.pone.0212785. eCollection 2019. PLoS One. 2019. PMID: 30893319 Free PMC article. - Sumatriptan plus naproxen for acute migraine attacks in adults.
Law S, Derry S, Moore RA. Law S, et al. Cochrane Database Syst Rev. 2013 Oct 21;(10):CD008541. doi: 10.1002/14651858.CD008541.pub2. Cochrane Database Syst Rev. 2013. PMID: 24142431 Updated. Review.
Cited by
- Improving medication adherence in migraine treatment.
Seng EK, Rains JA, Nicholson RA, Lipton RB. Seng EK, et al. Curr Pain Headache Rep. 2015 Jun;19(6):24. doi: 10.1007/s11916-015-0498-8. Curr Pain Headache Rep. 2015. PMID: 26040703 Review. - Headache-Specific Locus of Control and Migraine-Related Quality of Life: Understanding the Role of Anxiety.
Grinberg AS, Seng EK. Grinberg AS, et al. Int J Behav Med. 2017 Feb;24(1):136-143. doi: 10.1007/s12529-016-9587-2. Int J Behav Med. 2017. PMID: 27488417 Free PMC article. - Team players against headache: multidisciplinary treatment of primary headaches and medication overuse headache.
Gaul C, Visscher CM, Bhola R, Sorbi MJ, Galli F, Rasmussen AV, Jensen R. Gaul C, et al. J Headache Pain. 2011 Oct;12(5):511-9. doi: 10.1007/s10194-011-0364-y. Epub 2011 Jul 21. J Headache Pain. 2011. PMID: 21779789 Free PMC article. Review. - Cognitive Behavioral Therapy for Pediatric Headache and Migraine: Why to Prescribe and What New Research Is Critical for Advancing Integrated Biobehavioral Care.
Kroon Van Diest AM, Powers SW. Kroon Van Diest AM, et al. Headache. 2019 Feb;59(2):289-297. doi: 10.1111/head.13438. Epub 2018 Nov 16. Headache. 2019. PMID: 30444269 Free PMC article. Review. - Multimodal Migraine Management and the Pursuit of Migraine Freedom: A Narrative Review.
Blumenfeld AM, Lipton RB, Silberstein S, Tepper SJ, Charleston L 4th, Landy S, Kuruvilla DE, Manack Adams A. Blumenfeld AM, et al. Neurol Ther. 2023 Oct;12(5):1533-1551. doi: 10.1007/s40120-023-00529-x. Epub 2023 Aug 5. Neurol Ther. 2023. PMID: 37542624 Free PMC article. Review.
References
- Stovner LJ, Hagen K, Jensen R, Katsarava Z, Lipton RB, Scher AI, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007;27:193-210. - PubMed
- Lipton R, Stewart A, Diamond S, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:646-57. - PubMed
- Lipton R, Bigal M, Diamond M, Freitag F, Reed M, Stewart W. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68:343-9. - PubMed
- Von Korff M, Stewart W, Simon D, Lipton B. Migraine and reduced work performance—a population-based diary study. Neurology 1998;50:1741-5. - PubMed
- Terwindt G, Ferrari M, Tihuis M, Groenen S, Picavet H, Launer L. The impact of migraine on quality of life in the general population: the GEM study. Neurology 2000;55:624-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous