Microglial interactions with synapses are modulated by visual experience - PubMed (original) (raw)
Microglial interactions with synapses are modulated by visual experience
Marie-Ève Tremblay et al. PLoS Biol. 2010.
Abstract
Microglia are the immune cells of the brain. In the absence of pathological insult, their highly motile processes continually survey the brain parenchyma and transiently contact synaptic elements. Aside from monitoring, their physiological roles at synapses are not known. To gain insight into possible roles of microglia in the modification of synaptic structures, we used immunocytochemical electron microscopy, serial section electron microscopy with three-dimensional reconstructions, and two-photon in vivo imaging to characterize microglial interactions with synapses during normal and altered sensory experience, in the visual cortex of juvenile mice. During normal visual experience, most microglial processes displayed direct apposition with multiple synapse-associated elements, including synaptic clefts. Microglial processes were also distinctively surrounded by pockets of extracellular space. In terms of dynamics, microglial processes localized to the vicinity of small and transiently growing dendritic spines, which were typically lost over 2 d. When experience was manipulated through light deprivation and reexposure, microglial processes changed their morphology, showed altered distributions of extracellular space, displayed phagocytic structures, apposed synaptic clefts more frequently, and enveloped synapse-associated elements more extensively. While light deprivation induced microglia to become less motile and changed their preference of localization to the vicinity of a subset of larger dendritic spines that persistently shrank, light reexposure reversed these behaviors. Taken together, these findings reveal different modalities of microglial interactions with synapses that are subtly altered by sensory experience. These findings suggest that microglia may actively contribute to the experience-dependent modification or elimination of a specific subset of synapses in the healthy brain.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Figure 1. Ultrastructural interactions between microglia and synapses during normal sensory experience.
(A–C) EM images showing IBA1-immunostained microglial (m+) cell bodies (A), as well as large (B) and small (C) processes, surrounded by extended extracellular space (asterisks) and contacting axon terminals (blue), dendritic spines (pink), perisynaptic astrocytes (green), and synaptic clefts (arrowheads). d, dentrite; N, nucleus; p, perikaryon. Scale bars = 250 nm. (D) EM image showing extended microglia-associated extracellular spaces (asterisks) after glutaraldehyde instead of acrolein fixation. The unlabeled microglial process (m) makes direct contacts with dendritic spines (s) and axon terminals (t), and displays an inclusion (in), as well as a clathrin-coated pit (black arrow) at the site of contact with a spine. Scale bar = 250 nm. (E) Extracellular space areas with or without contact with IBA1-positive microglial process (mean ± SEM). **, p<0.01. (F) Correlation between the areas of microglial processes and associated extracellular space (r = 0.48; p<0.0001). See also Figure S1 and Tables S1 and S2.
Figure 2. 3-D reconstructions of the ultrastructural interactions between microglia and synapses during normal sensory experience.
(A) SSEM image showing a microglia (beige) contiguous to a neuronal perikaryon (p), with its associated extracellular space (asterisks) and contacted synapse-associated elements. Color scheme as in Figure 1. in, inclusion. (B) Partial 3-D reconstruction of the microglial proximal process (P; taupe) cut in transverse. Purple indicates the inclusion. Both processes simultaneously contact multiple axon terminals (blue), dendritic spines (red), and perisynaptic astrocytic processes (green), and are distinctively surrounded by extracellular space pockets of various size and shape (white; black arrows). (C) Additional view showing only microglia, dendritic spines, and extracellular space. (D and E) Insets illuminating the 3-D geometry of the distal protrusion and its structural relationships with one axon terminal (t1), two dendritic spines (s1 and s2; postsynaptic density in dark red), and a pocket of extracellular space (white), which are partially reconstructed. For clarity, an astrocytic process was removed from the scene. Scale bars = 250 nm. See also Figures S2, S3, S4 and Table S3.
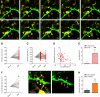
Figure 3. Structural/dynamic interactions between microglia and dendritic spines during normal sensory experience in vivo.
(A) Time-lapse images showing three dendritic spines (green; red arrowheads) contacted by microglia (yellow; white arrowheads) over 45 min. Scale bar = 5 µm. (B) Dendritic spine size changes during microglial contact. (C) Dendritic spine size before, during, and after contact. (D) Correlation between the initial dendritic spine size and the change in spine size during contact. (E) Average size changes in the presence versus in the absence of microglial contact for large and small dendritic spines (mean ± SEM). (F) Change in small dendritic spine size during microglial contact. (G) Images from chronic experiments showing the elimination of a dendritic spine that had been contacted by a microglial process over 2 d (white arrowhead during the contact; orange arrowhead after the contact), while the other contacted (white arrowheads during the contact; red arrowheads after the contact) and non-contacted (red arrowheads) spines remain stable. Scale bar = 5 µm. (H) Proportion of dendritic spines eliminated over 2 d in spine populations contacted and not contacted by microglia during the first imaging session (mean ± SEM). Values in (B), (C), and (F) were normalized to the first condition, and values in (D) were normalized to the size of the largest spine. au, arbitrary units. *, p<0.05; **, p<0.01. See also Figures S5, S6, S7, S8 and Videos S1 and S4.
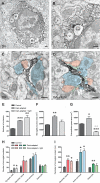
Figure 4. Ultrastructural relationships between microglia and synapses during altered visual experience.
(A and B) EM image from a DA animal showing multiple cellular inclusions (in) in an IBA1-positive microglial perikaryon (m+). (B) shows a magnified view of the boxed region in (A). One inclusion resembles a dendritic spine (“s”) receiving a synapse from an axon terminal (“t”), while the other inclusion contains an accumulation of cellular membranes (“cm”). a, perisynaptic astrocyte; N, nucleus; s, dendritic spine; t, axon terminal. Scale bars = 250 nm. (C and D) EM images taken in DA (C) and DA+light (D) animals displaying two “spindly” microglial processes making multiple contacts with synapse-associated elements, including synaptic clefts (white arrowheads). Note that the process in (C) is surrounded by extended extracellular space (asterisks) in contrast with the process in (D). Color scheme as in Figure 1. d, dendrite. Scale bars = 250 nm. (E) Change in the total number of cellular inclusions for 50 IBA1-positive microglial processes during DA and DA+light (mean ± SEM). (F and G) Change in microglial process area and microglia-associated extracellular space area for 50 IBA1-positive microglial processes during DA and DA+light (mean ± SEM). (H and I) Total number of contacts and average perimeter of contact between 50 IBA1-positive microglial processes and every synapse-associated element (mean ± SEM). *, p<0.05; **, p<0.01; ***, p<0.001. Black asterisks refer to comparisons with control animals and grey asterisks to comparisons with DA animals. See also Figures S9 and S10 and Tables S1 and S2.
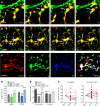
Figure 5. Changes in microglial behavior during altered visual experience.
(A) Time-lapse images taken in a DA animal showing a microglial process that terminates into a structurally stable phagocytic cup (white arrow), while another process dynamically contacts two dendritic spines (red arrowheads before the contact; white arrowheads during the contact). Scale bar = 5 µm. (B) Time-lapse images showing another example of a structurally stable phagocytic cup (white arrow) in a DA animal. Scale bar = 10 µm. (C) Time-lapse images showing the motility of microglia during normal visual experience over three time points, 0, 5, and 25 min. Images from each time point were colored in red, green, and blue, respectively, and then merged to reveal microglia-associated pixels that were unchanged throughout the three time points (stable; white), changed in one of the three time points (dynamic; yellow and fuchsia), or changed in two of the three time points (highly dynamic; red, green, or blue). Scale bar = 10 µm. (D) Change in microglial motility index during DA and DA+light, measured as the proportion of the pixels that differed between images of a single microglia taken 5 or 25 min apart (mean±SEM). Black asterisks refer to comparisons with control animals, and green or blue asterisks to comparisons with DA animals. (E) Initial size of non-contacted and contacted dendritic spines in control, DA, and DA+light animals (mean ± SEM). Black asterisks refer to comparisons with non-contacted spines in control animals, dark grey asterisks to comparisons with non-contacted spines in DA animals, and light grey asterisks to comparisons with non-contacted spines in animals reexposed to light. (F) Dendritic spine size before, during, and after microglial contact in DA animals (left) and DA+light animals (right). Data were normalized to the first condition for presentation purposes. *, p<0.05; **, p<0.01; ***, p<0.001. See also Figures S11 and S12, as well as Videos S2, S3, S5, and S6.
Similar articles
- Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals.
Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Wake H, et al. J Neurosci. 2009 Apr 1;29(13):3974-80. doi: 10.1523/JNEUROSCI.4363-08.2009. J Neurosci. 2009. PMID: 19339593 Free PMC article. - The role of microglia at synapses in the healthy CNS: novel insights from recent imaging studies.
Tremblay MÈ. Tremblay MÈ. Neuron Glia Biol. 2011 Feb;7(1):67-76. doi: 10.1017/S1740925X12000038. Epub 2012 Mar 15. Neuron Glia Biol. 2011. PMID: 22418067 Review. - A role for microglia in synaptic plasticity?
Tremblay MÈ, Majewska AK. Tremblay MÈ, et al. Commun Integr Biol. 2011 Mar;4(2):220-2. doi: 10.4161/cib.4.2.14506. Commun Integr Biol. 2011. PMID: 21655446 Free PMC article. - Sensory Experience Engages Microglia to Shape Neural Connectivity through a Non-Phagocytic Mechanism.
Cheadle L, Rivera SA, Phelps JS, Ennis KA, Stevens B, Burkly LC, Lee WA, Greenberg ME. Cheadle L, et al. Neuron. 2020 Nov 11;108(3):451-468.e9. doi: 10.1016/j.neuron.2020.08.002. Epub 2020 Sep 14. Neuron. 2020. PMID: 32931754 Free PMC article. - Snapshot of microglial physiological functions.
Verkhratsky A, Sun D, Tanaka J. Verkhratsky A, et al. Neurochem Int. 2021 Mar;144:104960. doi: 10.1016/j.neuint.2021.104960. Epub 2021 Jan 15. Neurochem Int. 2021. PMID: 33460721 Review.
Cited by
- Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells.
Varvel NH, Grathwohl SA, Baumann F, Liebig C, Bosch A, Brawek B, Thal DR, Charo IF, Heppner FL, Aguzzi A, Garaschuk O, Ransohoff RM, Jucker M. Varvel NH, et al. Proc Natl Acad Sci U S A. 2012 Oct 30;109(44):18150-5. doi: 10.1073/pnas.1210150109. Epub 2012 Oct 15. Proc Natl Acad Sci U S A. 2012. PMID: 23071306 Free PMC article. - The Impact of Microglia on Neurodevelopment and Brain Function in Autism.
Luo Y, Wang Z. Luo Y, et al. Biomedicines. 2024 Jan 17;12(1):210. doi: 10.3390/biomedicines12010210. Biomedicines. 2024. PMID: 38255315 Free PMC article. Review. - Time-lapse Whole-field Fluorescence Imaging of Microglia ProcessesMotility in Acute Mouse Hippocampal Slices and Analysis.
Basilico B, Cortese B, Ratano P, Angelantonio SD, Ragozzino D. Basilico B, et al. Bio Protoc. 2019 Apr 20;9(8):e3220. doi: 10.21769/BioProtoc.3220. eCollection 2019 Apr 20. Bio Protoc. 2019. PMID: 33655009 Free PMC article. - Identification of a chronic non-neurodegenerative microglia activation state in a mouse model of peroxisomal β-oxidation deficiency.
Verheijden S, Beckers L, Casazza A, Butovsky O, Mazzone M, Baes M. Verheijden S, et al. Glia. 2015 Sep;63(9):1606-20. doi: 10.1002/glia.22831. Epub 2015 Apr 2. Glia. 2015. PMID: 25846981 Free PMC article. - Inflammatory responses to alcohol in the CNS: nuclear receptors as potential therapeutics for alcohol-induced neuropathologies.
Kane CJ, Drew PD. Kane CJ, et al. J Leukoc Biol. 2016 Nov;100(5):951-959. doi: 10.1189/jlb.3MR0416-171R. Epub 2016 Jul 26. J Leukoc Biol. 2016. PMID: 27462100 Free PMC article. Review.
References
- Marin-Teva J. L, Dusart I, Colin C, Gervais A, van Rooijen N, et al. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. - PubMed
- Frade J. M, Barde Y. A. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. - PubMed
- Upender M. B, Naegele J. R. Activation of microglia during developmentally regulated cell death in the cerebral cortex. Dev Neurosci. 1999;21:491–505. - PubMed
- Cuadros M. A, Navascues J. The origin and differentiation of microglial cells during development. Prog Neurobiol. 1998;56:173–189. - PubMed
- Hanisch U. K, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources