Metabolic and hedonic drives in the neural control of appetite: who is the boss? - PubMed (original) (raw)
Review
Metabolic and hedonic drives in the neural control of appetite: who is the boss?
Hans-Rudolf Berthoud. Curr Opin Neurobiol. 2011 Dec.
Abstract
Obesity is on the rise in all developed countries, and a large part of this epidemic has been attributed to excess caloric intake, induced by ever present food cues and the easy availability of energy dense foods in an environment of plenty. Clearly, there are strong homeostatic regulatory mechanisms keeping body weight of many individuals exposed to this environment remarkably stable over their adult life. Other individuals, however, seem to eat not only because of metabolic need, but also because of excessive hedonic drive to make them feel better and relieve stress. In the extreme, some individuals exhibit addiction-like behavior toward food, and parallels have been drawn to drug and alcohol addiction. However, there is an important distinction in that, unlike drugs and alcohol, food is a daily necessity. Considerable advances have been made recently in the identification of neural circuits that represent the interface between the metabolic and hedonic drives of eating. We will cover these new findings by focusing first on the capacity of metabolic signals to modulate processing of cognitive and reward functions in cortico-limbic systems (bottom-up) and then on pathways by which the cognitive and emotional brain may override homeostatic regulation (top-down).
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
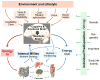
Fig. 1
Schematic diagram showing the major factors determining neural control of appetite and regulation of energy balance. The brain monitors the internal milieu through a number of hormonal and neural nutrient sensing mechanisms and is under constant influence of the environment and lifestyle through the senses and mainly the cognitive and emotional brain. The two streams of information are integrated to generate adaptive behavioral (food intake) and autonomic/endocrine responsses determining nutrient partitioning, energy expenditure, and overall energy balance. All of the peripheral and central signaling steps are subject to individual predisposition either through genetic, epigenetic, or non-genetic early life imprinting mechanisms.
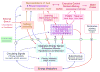
Fig. 2
Schematic diagram showing potential interactions between the so called “homeostatic” energy balance regulatory system (blue) and neural systems involved in external sensory information processing (yellowish-brown), reward processing (purple), and cognition and executive functions (red), collectively referred to as “hedonic systems”. Blue arrows indicate bottom-up modulation of hedonic systems by homeostatic signals. Broken blue lines represent circulating hormones, metabolites, and other factors; solid blue lines represent neural pathways. Red arrows indicate top-down modulation of homeostatic processes by hedonic drives. See text for discussion of specific interactive pathways.
Similar articles
- Metabolic vs. hedonic obesity: a conceptual distinction and its clinical implications.
Yu YH, Vasselli JR, Zhang Y, Mechanick JI, Korner J, Peterli R. Yu YH, et al. Obes Rev. 2015 Mar;16(3):234-47. doi: 10.1111/obr.12246. Epub 2015 Jan 14. Obes Rev. 2015. PMID: 25588316 Free PMC article. Review. - Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals.
Zheng H, Lenard NR, Shin AC, Berthoud HR. Zheng H, et al. Int J Obes (Lond). 2009 Jun;33 Suppl 2(Suppl 2):S8-13. doi: 10.1038/ijo.2009.65. Int J Obes (Lond). 2009. PMID: 19528982 Free PMC article. Review. - Interactions between metabolic, reward and cognitive processes in appetite control: Implications for novel weight management therapies.
Higgs S, Spetter MS, Thomas JM, Rotshtein P, Lee M, Hallschmid M, Dourish CT. Higgs S, et al. J Psychopharmacol. 2017 Nov;31(11):1460-1474. doi: 10.1177/0269881117736917. Epub 2017 Oct 26. J Psychopharmacol. 2017. PMID: 29072515 Free PMC article. Review. - Food for Thought: Reward Mechanisms and Hedonic Overeating in Obesity.
Lee PC, Dixon JB. Lee PC, et al. Curr Obes Rep. 2017 Dec;6(4):353-361. doi: 10.1007/s13679-017-0280-9. Curr Obes Rep. 2017. PMID: 29052153 Review. - Functional brain imaging of appetite.
Dagher A. Dagher A. Trends Endocrinol Metab. 2012 May;23(5):250-60. doi: 10.1016/j.tem.2012.02.009. Epub 2012 Apr 5. Trends Endocrinol Metab. 2012. PMID: 22483361 Review.
Cited by
- Emotional eating, internet overuse, and alcohol intake among college students: a pilot study with virtual reality.
Marchena-Giráldez C, Carbonell-Colomer M, Bernabéu-Brotons E. Marchena-Giráldez C, et al. Front Nutr. 2024 Jun 18;11:1400815. doi: 10.3389/fnut.2024.1400815. eCollection 2024. Front Nutr. 2024. PMID: 38957869 Free PMC article. - Food Cravings and Obesity in Women with Polycystic Ovary Syndrome: Pathophysiological and Therapeutic Considerations.
Stefanaki K, Karagiannakis DS, Peppa M, Vryonidou A, Kalantaridou S, Goulis DG, Psaltopoulou T, Paschou SA. Stefanaki K, et al. Nutrients. 2024 Apr 3;16(7):1049. doi: 10.3390/nu16071049. Nutrients. 2024. PMID: 38613082 Free PMC article. Review. - Mindfulness, mental health, and motives for eating tasty foods when not in metabolic need.
Moore KG, Rice JD, Gampher JE, Boggiano MM. Moore KG, et al. Front Psychol. 2024 Jan 19;14:1308609. doi: 10.3389/fpsyg.2023.1308609. eCollection 2023. Front Psychol. 2024. PMID: 38314255 Free PMC article. - Case Reports of Binge Eating Patterns in the Recovery Phase of Anorexia Nervosa Patients With and Without Food Addiction.
Song Y, Park MJ, Choi HJ. Song Y, et al. Soa Chongsonyon Chongsin Uihak. 2024 Jan 1;35(1):66-74. doi: 10.5765/jkacap.230069. Soa Chongsonyon Chongsin Uihak. 2024. PMID: 38204743 Free PMC article. - Dynamical alterations of brain function and gut microbiome in weight loss.
Zhou J, Wu X, Xiang T, Liu F, Gao H, Tong L, Yan B, Li Z, Zhang C, Wang L, Ou L, Li Z, Wang W, Yang T, Li F, Ma H, Zhao X, Mi N, Yu Z, Lan C, Wang Q, Li H, Wang L, Wang X, Li Y, Zeng Q. Zhou J, et al. Front Cell Infect Microbiol. 2023 Dec 20;13:1269548. doi: 10.3389/fcimb.2023.1269548. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38173792 Free PMC article.
References
- Hoebel BG. Inhibition and disinhibition of self-stimulation and feeding: hypothalamic control and postingestional factors. J Comp Physiol Psychol. 1968;66:89–100. - PubMed
- Goldstone AP, de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, Durighel G, Hughes E, Waldman AD, Frost G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30:1625–1635. This fMRI study shows that when metabolically hungry, pictures of high calorie foods were more appealing and produced higher neural activation in cortico-limbic structures than pictures of low calorie foods, and subjective rating and brain responses were positively correlated. - PubMed
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. This paper is a brief summary of the highly influential theoretical framework of distinctive behavioral functions and underlying neural circuitries contributing to food reward behaviors. - PMC - PubMed
- Tindell AJ, Smith KS, Berridge KC, Aldridge JW. Dynamic computation of incentive salience: “wanting” what was never “liked”. J Neurosci. 2009;29:12220–12228. In a model of salt depletion in rats the authors show that mesocorticolimbic systems including the ventral pallidum can compute “wanting’ of a nutrient de novo by integrating novel physiological signals with a cue’s preexisting associations to an outcome that lacked hedonic value. - PMC - PubMed
- Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology. 2004;145:839–847. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DK071082/DK/NIDDK NIH HHS/United States
- R01 DK047348-13/DK/NIDDK NIH HHS/United States
- R01 DK071082-04/DK/NIDDK NIH HHS/United States
- R01 DK047348/DK/NIDDK NIH HHS/United States
- R01 DK071082-04S1/DK/NIDDK NIH HHS/United States
- R01 DK047348-17/DK/NIDDK NIH HHS/United States
- R01 DK071082-04S2/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical