MG63 osteoblast-like cells exhibit different behavior when grown on electrospun collagen matrix versus electrospun gelatin matrix - PubMed (original) (raw)
MG63 osteoblast-like cells exhibit different behavior when grown on electrospun collagen matrix versus electrospun gelatin matrix
Shiao-Wen Tsai et al. PLoS One. 2012.
Abstract
Electrospinning is a simple and efficient method of fabricating a non-woven polymeric nanofiber matrix. However, using fluorinated alcohols as a solvent for the electrospinning of proteins often results in protein denaturation. TEM and circular dichroism analysis indicated a massive loss of triple-helical collagen from an electrospun collagen (EC) matrix, and the random coils were similar to those found in gelatin. Nevertheless, from mechanical testing we found the Young's modulus and ultimate tensile stresses of EC matrices were significantly higher than electrospun gelatin (EG) matrices because matrix stiffness can affect many cell behaviors such as cell adhesion, proliferation and differentiation. We hypothesize that the difference of matrix stiffness between EC and EG will affect intracellular signaling through the mechano-transducers Rho kinase (ROCK) and focal adhesion kinase (FAK) and subsequently regulates the osteogenic phenotype of MG63 osteoblast-like cells. From the results, we found there was no significant difference between the EC and EG matrices with respect to either cell attachment or proliferation rate. However, the gene expression levels of OPN, type I collagen, ALP, and OCN were significantly higher in MG63 osteoblast-like cells grown on the EC than in those grown on the EG. In addition, the phosphorylation levels of Y397-FAK, ERK1/2, BSP, and OPN proteins, as well as ALP activity, were also higher on the EC than on the EG. We further inhibited ROCK activation with Y27632 during differentiation to investigate its effects on matrix-mediated osteogenic differentiation. Results showed the extent of mineralization was decreased with inhibition after induction. Moreover, there is no significant difference between EC and EG. From the results of the protein levels of phosphorylated Y397-FAK, ERK1/2, BSP and OPN, ALP activity and mineral deposition, we speculate that the mechanism that influences the osteogenic differentiation of MG63 osteoblast-like cells on EC and EG is matrix stiffness and via ROCK-FAK-ERK1/2.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Electron microscopic images of electrospun collagen matrix (EC) and electrospun gelatin matrix (EG).
Scanning electron microscopic images of (A) electrospun collagen matrix (EC), and (B) electrospun gelatin matrix (EG) at 2,500× magnification. The EC nanofibers had an average diameter of 692±214 nm, and the EG nanofibers had an average diameter of 573±368 nm. Scale bar is 20 µm. Transmission electron microscopic images of (C) electrospun collagen matrix, (D) electrospun gelatin matrix and (E) self-assembled collagen matrix. Scale bar is 0.2 µm. EC nanofibers did not exhibit the characteristic D-periods pattern that was apparent for the native collagen molecules self-assembled into collagen fibrils. Self-assembled collagen was formed by dialyzed collagen solution (in acetic acid) against 0.02 M phosphate buffered-saline (PBS, pH 7) at 4°C. Fibril formation (self-assembled) was initiated by warming the mixture to 37°C for 4 hours.
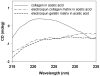
Figure 2. CD spectra of the different matrix preparations.
Circular dichroism spectra of the acid-solubilized materials plotted as mean residue ellipticity (mdeg×cm2×dmol−1)] vs. wavelength (nm). The triple-helical structure of native collagen have a characteristic positive peak at around 220 nm. The EC curve indicated a massive loss of triple-helical structure and similar to EG.
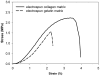
Figure 3. The tensile stress-strain curve of an electrospun collagen matrix and an electrospun gelatin matrix.
The Young's modulus of the EC and EG were 94.29±15.18 and 71.88±21.10 MPa, respectively. The ultimate tensile stresses the EC and EG were 1.93±0.37 and 0.93±0.46 MPa, respectively.
Figure 4. Quantification of MG63 osteoblast-like cells on EC and EG matrices.
WST assay quantifying cell attachment and proliferation on electrospun scaffolds of collagen and gelatin. (A) The attachment of MG63 osteoblast-like cells on various matrices after culturing for up to 4 hours. (B) The viability of MG63 osteoblast-like cells on various matrices after culturing for up to14 days. Data are presented as mean ± SD, n = 4. Statistical analysis was compared between EC and EG. There were no significant differences in cellular attachment or proliferation between EC and EG (_p_>0.05).
Figure 5. Different cellular morphology of cells on EC and EG matrices.
Fluorescent microscopic micrographs of MG63 osteoblast-like cells cultured on matrix for 4 hours. (A) electrospun collagen matrix. (B) electrospun gelatin matrix. Cells cultured on EC showed well-organized F-actin stress fibers. On the other hand, cells cultured on EG showed with numerous, very thin and long filopodia. Cytoskeletal F-actin is stained green with FITC and cell nuclei are stained blue with DAPI. (white arrow: filopodia, Scale bar = 10 µm)
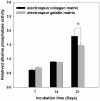
Figure 6. Analysis of alkaline phosphatase activity of cells on EC and EG matrices.
The alkaline phosphatase activity of cells was found to be significantly higher on EC than on EG on day 21. Data are presented as mean ± SD, n = 4. Statistical analysis was compared between EC and EG. (*) denotes a significant difference (p<0.05).
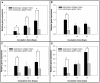
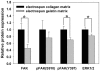
Figure 7. Matrices regulate the bone-associated genes expressed by MG63 osteoblast-like cells.
Real–time PCR analyses of bone-associated genes expressed by MG63 osteoblast-like cells on various matrix. (A) β-actin levels, (B) type I collagen levels, (C) OPN levels and (D) OCN levels after normalization to 18S ribosomal RNA levels. Data are shown as the fold change relative to the electrospun collagen matrix after 7 days of incubation. On day 7, the cells showed significantly higher expression levels of β-actin, type I collagen, OPN and OCN when grown on EC. And, the expression levels of β-actin, OPN and OCN were significantly higher on day 21 when grown on EC. Data are presented as mean ± SD, n = 4. Statistical analysis was compared between EC and EG. (*) denotes a significant difference (p<0.05).
Figure 8. Effects of matrix on FAK and phospho-FAK and ERK1/2 activation in MG63 osteoblasts-like cell.
Densitometric quantitation of the protein bands was performed after normalizing to nucleophosmin B23 levels. (Figure S1 shown the images of full western blots). Data are shown as the fold change of the corresponding 2 h of incubation on the EC. The total FAK levels expressed by MG63 osteoblast-like cells were significant higher in cells grown on EC than in those grown on EG. Moreover, the level of FAK phosphorylation at tyrosine 397 was lower in cells grown on EG than in those grown on EC. Data are presented as mean ± SD, n = 3. Statistical analysis was compared between EC and EG. (*) denotes a significant difference (p<0.05).
Figure 9. Effects of matrix on BSP and OPN protein expression in MG63 osteoblasts-like cell.
Western blot analysis for BSP and OPN proteins in cells cultured for (A) 14 and (B) 21 days. Densitometric quantitation of the OPN and BSP protein band were performed after normalizing to nucleophosmin B23 levels (Figure S2 shown the images of full western blots). Data are shown as the fold change of the corresponding on the electrospun collagen matrix. The expression level of BSP and OPN proteins showed a significant increase in cells grown on EC at 21 days. Data represent mean ± SD, n = 3. Statistical analysis was compared between EC and EG. (*) denotes a significant difference (p<0.05).
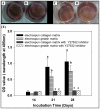
Figure 10. Effects of matrix on bone mineralization in MG63 osteoblasts-like cell.
Optical images of ARS staining for mineralization in MG63 osteoblast-like cells for day 28 (A) EC, (B) EG. (C) EC with 10 µM Y27632, (D) EG with 10 µM Y27632. Quantification of mineral deposition by Alizarin Red-S staining. Data represent mean ± SD, n = 4. Statistical analysis was compared between EC and EG with or without Y27632. Different letters represent significance at p<0.05 by non-parametric Mann-Whitney U test.
Similar articles
- Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells.
Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK. Shih YR, et al. J Bone Miner Res. 2011 Apr;26(4):730-8. doi: 10.1002/jbmr.278. J Bone Miner Res. 2011. PMID: 20939067 - Collagen I induces the expression of alkaline phosphatase and osteopontin via independent activations of FAK and ERK signalling pathways.
Viale-Bouroncle S, Gosau M, Morsczeck C. Viale-Bouroncle S, et al. Arch Oral Biol. 2014 Dec;59(12):1249-55. doi: 10.1016/j.archoralbio.2014.07.013. Epub 2014 Jul 24. Arch Oral Biol. 2014. PMID: 25150530 - The Effect of Strontium-Substituted Hydroxyapatite Nanofibrous Matrix on Osteoblast Proliferation and Differentiation.
Tsai SW, Hsu YW, Pan WL, Hsu FY. Tsai SW, et al. Membranes (Basel). 2021 Aug 14;11(8):624. doi: 10.3390/membranes11080624. Membranes (Basel). 2021. PMID: 34436387 Free PMC article. - ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix.
Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. Wozniak MA, et al. J Cell Biol. 2003 Nov 10;163(3):583-95. doi: 10.1083/jcb.200305010. J Cell Biol. 2003. PMID: 14610060 Free PMC article. - Fiber diameters control osteoblastic cell migration and differentiation in electrospun gelatin.
Sisson K, Zhang C, Farach-Carson MC, Chase DB, Rabolt JF. Sisson K, et al. J Biomed Mater Res A. 2010 Sep 15;94(4):1312-20. doi: 10.1002/jbm.a.32756. J Biomed Mater Res A. 2010. PMID: 20694999
Cited by
- Fabrication and Characteristics of PCL Membranes Containing Strontium-Substituted Hydroxyapatite Nanofibers for Guided Bone Regeneration.
Tsai SW, Yu WX, Hwang PA, Hsu YW, Hsu FY. Tsai SW, et al. Polymers (Basel). 2019 Oct 27;11(11):1761. doi: 10.3390/polym11111761. Polymers (Basel). 2019. PMID: 31717839 Free PMC article. - A Comparative Study on Physicochemical Properties and In Vitro Biocompatibility of Sr-Substituted and Sr Ranelate-Loaded Hydroxyapatite Nanoparticles.
Stipniece L, Ramata-Stunda A, Vecstaudza J, Kreicberga I, Livkisa D, Rubina A, Sceglovs A, Salma-Ancane K. Stipniece L, et al. ACS Appl Bio Mater. 2023 Dec 18;6(12):5264-5281. doi: 10.1021/acsabm.3c00539. Epub 2023 Dec 1. ACS Appl Bio Mater. 2023. PMID: 38039078 Free PMC article. - The effect of substrate stiffness, thickness, and cross-linking density on osteogenic cell behavior.
Mullen CA, Vaughan TJ, Billiar KL, McNamara LM. Mullen CA, et al. Biophys J. 2015 Apr 7;108(7):1604-1612. doi: 10.1016/j.bpj.2015.02.022. Biophys J. 2015. PMID: 25863052 Free PMC article. - Radially patterned transplantable biodegradable scaffolds as topographically defined contact guidance platforms for accelerating bone regeneration.
Gwon Y, Park S, Kim W, Han T, Kim H, Kim J. Gwon Y, et al. J Biol Eng. 2021 Mar 22;15(1):12. doi: 10.1186/s13036-021-00263-8. J Biol Eng. 2021. PMID: 33752709 Free PMC article. - Evaluation of insulin medium or chondrogenic medium on proliferation and chondrogenesis of ATDC5 cells.
Yao Y, Zhai Z, Wang Y. Yao Y, et al. Biomed Res Int. 2014;2014:569241. doi: 10.1155/2014/569241. Epub 2014 Apr 10. Biomed Res Int. 2014. PMID: 24812622 Free PMC article.
References
- Schnaper HW, Kleinman HK. Regulation of cell function by extracellular matrix. Pediatr Nephrol. 1993;7:96–104. - PubMed
- Weiss P. Experiments on cell and axon orientation in vitro: The role of colloidal exudates in tissue organization. J Exp Zool. 1945;68:353–448. - PubMed
- Dalby MJ, Riehle MO, Johnstone H, Affrossman S, Curtis AS. In vitro reaction of endothelial cells to polymer demixed nanotopography. Biomaterials. 2002;23:2945–2954. - PubMed
- Dalby MJ, Childs S, Riehle MO, Johnstone HJ, Affrossman S, et al. Fibroblast reaction to island topography: changes in cytoskeleton and morphology with time. Biomaterials. 2003;24:927–935. - PubMed
- Tsai SW, Chen CC, Chen PL, Hsu FY. Influence of topography of nanofibrils of three-dimensional collagen gel beads on the phenotype, proliferation, and maturation of osteoblasts. J Biomed Mater Res A. 2009;91:985–993. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous