Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence - PubMed (original) (raw)
Clinical Trial
. 2012 Jun 21;119(25):6145-54.
doi: 10.1182/blood-2011-12-395970. Epub 2012 May 4.
Bita Sahaf, Balasubramanian Narasimhan, George L Chen, Carol D Jones, Robert Lowsky, Judith A Shizuru, Laura J Johnston, Ginna G Laport, Wen-Kai Weng, Jonathan E Benjamin, Joanna Schaenman, Janice Brown, Jessica Ramirez, James L Zehnder, Robert S Negrin, David B Miklos
Affiliations
- PMID: 22563089
- PMCID: PMC3383022
- DOI: 10.1182/blood-2011-12-395970
Clinical Trial
Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence
Sally Arai et al. Blood. 2012.
Abstract
B cells are involved in the pathogenesis of chronic GVHD (cGVHD). We hypothesized that prophylactic anti-B-cell therapy delivered 2 months after transplantation would decrease allogeneic donor B-cell immunity and possibly the incidence of cGVHD. Therefore, in the present study, patients with high-risk chronic lymphocytic leukemia (n = 22) and mantle-cell lymphoma (n = 13) received a total lymphoid irradiation of 80 cGy for 10 days and antithymocyte globulin 1.5 mg/kg/d for 5 days. Rituximab (375 mg/m(2)) was infused weekly on days 56, 63, 70, and 77 after transplantation. The incidence of acute GVHD was 6%. The cumulative incidence of cGVHD was 20%. Nonrelapse mortality was 3%. Rituximab treatment after allogeneic transplantation significantly reduced B-cell allogeneic immunity, with complete prevention of alloreactive H-Y Ab development in male patients with female donors (P = .01). Overall survival and freedom from progression at 4 years for chronic lymphocytic leukemia patients were 73% and 47%, respectively; for mantle-cell lymphoma patients, they were 69% and 53%, respectively.
Trial registration: ClinicalTrials.gov NCT00186628.
Figures
Figure 1
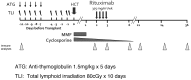
Trial schema. RIC using 80 cGy TLI for 10 days and ATG 1.5 mg/kg on days 1-5, followed by peripheral blood progenitor cell infusion on day 0. Rituximab infusion (375 mg/m2) was infused on days 56, 63, 70, 77. Cyclosporine and mycophenolate mofetil were used as primary GVHD prophylaxis. Triangles indicate time points for peripheral blood immune analyses.
Figure 2
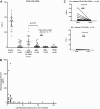
B-cell reconstitution and rituximab quantification. (A) Blood CD19+CD5−CD23− B-cell quantification. Blood samples collected from HLA identical donors (○), and study subjects (●) before TLI-ATG conditioning and 56 days after alloHCT were FACS analyzed to quantify CD19+ B cells. Recipient MCL and CLL cancer cells were excluded from this donor B-cell quantification by excluding CD5+ or CD23+ cells. Blood CD19+CD5−CD23− B-cell quantification performed 90 and 180 days after alloHCT showed limited donor B-cell recovery after rituximab. For comparison, donor B-cell recovery 56 days after TLI-ATG alloHCT was determined in 19 patients who never received rituximab (▴) to control for passive transmission of rituximab infused before HCT. (B) Rituximab infused 6 months or less before HCT is detected by ELISA at transplantation. Blood collected immediately before conditioning was measured by ELISA for rituximab concentration (μg/mL; y-axis). Each pre-HCT rituximab level was related to the number of months since their last rituximab infusion (x-axis). (C) CLL decreases following rituximab infusion 56 days after alloHCT. Immunophenotyping detected persistent CD19+CD5+CD23+ CLL cells 56 days after HCT in most CLL patients. Ten of 16 (63%) patients had > 10 CD19+CD5+CD23+ cells/μL of peripheral blood. After rituximab infusion, only 4 of 20 had CLL detected by flow cytometry on day 90. Twelve MCL patients had negligible CD19+CD5+CD23− cells in the blood measured on both days 56 and 90.
Figure 3
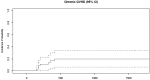
Only 20% of patients receiving rituximab prophylaxis developed cGVHD. The cumulative incidence of cGVHD at 4 years was 20% (95% CI, 6%-34%).
Figure 4
Rituximab prophylaxis prevents H-Y allogeneic Ab development. Blood IgGs against 5 H-Y antigens were determined by ELISA in 25 F → M HCT patients who never received rituximab after TLI-ATG (left panel) and 10 study patients treated with rituximab on days 56, 63, 70, and 77. These heat maps show that no alloreactive H-Y Abs developed in study patients receiving rituximab 2 months after TLI-ATG alloHCT, whereas 1 or more H-Y Abs developed in 56% (14 of 25) of patients receiving TLI-ATG without posttransplantation rituximab. Considering all patients who survived 9 months, rituximab prophylaxis prevents H-Y Ab development (P = .01).
Figure 5
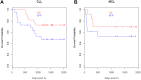
Overall survival after rituximab prophylaxis exceeds 70%. For the CLL patients, the 4-year overall survival was 73% (95% CI, 57%-94%) and freedom from progression was 47% (95% CI, 30%-75%). For the MCL patients, the 4-year overall survival was 69% (95% CI, 48%-99%) and freedom from progression was 53% (95% CI, 31%-89%).
Similar articles
- Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality.
Khouri IF, Saliba RM, Giralt SA, Lee MS, Okoroji GJ, Hagemeister FB, Korbling M, Younes A, Ippoliti C, Gajewski JL, McLaughlin P, Anderlini P, Donato ML, Cabanillas FF, Champlin RE. Khouri IF, et al. Blood. 2001 Dec 15;98(13):3595-9. doi: 10.1182/blood.v98.13.3595. Blood. 2001. PMID: 11739162 Clinical Trial. - Rituximab, fludarabine, and total body irradiation as conditioning regimen before allogeneic hematopoietic stem cell transplantation for advanced chronic lymphocytic leukemia: long-term prospective multicenter study.
Michallet M, Socié G, Mohty M, Sobh M, Bay JO, Morisset S, Labussière-Wallet H, Tabrizi R, Milpied N, Bordigoni P, El-Cheikh J, Blaise D. Michallet M, et al. Exp Hematol. 2013 Feb;41(2):127-33. doi: 10.1016/j.exphem.2012.10.008. Epub 2012 Oct 23. Exp Hematol. 2013. PMID: 23089183 Clinical Trial. - The effect of in vivo T cell depletion with alemtuzumab on reduced-intensity allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia.
Delgado J, Pillai S, Benjamin R, Caballero D, Martino R, Nathwani A, Lovell R, Thomson K, Perez-Simon JA, Sureda A, Kottaridis P, Vazquez L, Peggs K, Sierra J, Milligan D, Mackinnon S. Delgado J, et al. Biol Blood Marrow Transplant. 2008 Nov;14(11):1288-97. doi: 10.1016/j.bbmt.2008.09.001. Biol Blood Marrow Transplant. 2008. PMID: 18940684 - Rituximab for prevention and treatment of graft-versus-host disease.
Kharfan-Dabaja MA, Cutler CS. Kharfan-Dabaja MA, et al. Int J Hematol. 2011 May;93(5):578-585. doi: 10.1007/s12185-011-0855-2. Epub 2011 May 7. Int J Hematol. 2011. PMID: 21547615 Review. - Emerging role of CD20 blockade in allogeneic hematopoietic cell transplantation.
Kharfan-Dabaja MA, Bazarbachi A. Kharfan-Dabaja MA, et al. Biol Blood Marrow Transplant. 2010 Oct;16(10):1347-54. doi: 10.1016/j.bbmt.2010.01.005. Epub 2010 Jan 18. Biol Blood Marrow Transplant. 2010. PMID: 20083213 Review.
Cited by
- Recent advances and research progress regarding monoclonal antibodies for chronic graft-versus-host disease.
Huang S, Cheng X, Yang G, Huang R, Feng Y, Zeng L, Wu T, Song Q, Wang X, Zhang X. Huang S, et al. Heliyon. 2024 Sep 25;10(19):e38460. doi: 10.1016/j.heliyon.2024.e38460. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39403509 Free PMC article. Review. - Allogeneic haematopoietic cell transplantation for therapy-related myeloid neoplasms arising following treatment for lymphoma: a retrospective study on behalf of the Chronic Malignancies Working Party of the EBMT.
Nabergoj M, Eikema DJ, Koster L, Platzbecker U, Sockel K, Finke J, Kröger N, Forcade E, Nagler A, Eder M, Tischer J, Broers AEC, Kuball J, Wilson KMO, Hunault-Berger M, Collin M, Russo D, Corral LL, Helbig G, Mussetti A, Scheid C, Gurnari C, Raj K, Drozd-Sokolowska J, Yakoub-Agha I, Robin M, McLornan DP. Nabergoj M, et al. Bone Marrow Transplant. 2024 Mar;59(3):395-402. doi: 10.1038/s41409-023-02193-z. Epub 2024 Jan 9. Bone Marrow Transplant. 2024. PMID: 38195984 - Ofatumumab as part of reduced intensity conditioning in high risk B-cell lymphoma patients: final long-term analysis from a prospective multicenter Phase-II Trial.
Cabrero M, López-Corral L, Jarque I, de la Cruz-Vicente F, Pérez-López E, Valcárcel D, Sanz J, Espigado I, Ortí G, Martín-Calvo C, de la Serna J, Caballero D; Grupo Español de Trasplante Hematopoyético (GETH) and Grupo Español de Linfomas y Trasplante Autólogo (GELTAMO). Cabrero M, et al. Bone Marrow Transplant. 2024 Mar;59(3):359-365. doi: 10.1038/s41409-023-02171-5. Epub 2024 Jan 2. Bone Marrow Transplant. 2024. PMID: 38167647 Clinical Trial. - Planning GvHD preemptive therapy: risk factors, biomarkers, and prognostic scores.
Rozmus J, Levine JE, Schultz KR. Rozmus J, et al. Hematology Am Soc Hematol Educ Program. 2023 Dec 8;2023(1):149-154. doi: 10.1182/hematology.2023000425. Hematology Am Soc Hematol Educ Program. 2023. PMID: 38066854 Free PMC article. - Controversies and expectations for the prevention of GVHD: A biological and clinical perspective.
Watkins B, Williams KM. Watkins B, et al. Front Immunol. 2022 Nov 23;13:1057694. doi: 10.3389/fimmu.2022.1057694. eCollection 2022. Front Immunol. 2022. PMID: 36505500 Free PMC article. Review.
References
- Lönnqvist B, Aschan J, Ljungman P, Ringden O. Long-term cyclosporin therapy may decrease the risk of chronic graft-versus-host disease. Br J Haematol. 1990;74(4):547–548. - PubMed
- Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–2068. - PubMed
- Kansu E, Gooley T, Flowers ME, et al. Administration of cyclosporine for 24 months compared with 6 months for prevention of chronic graft-versus-host disease: a prospective randomized clinical trial. Blood. 2001;98(13):3868–3870. - PubMed
- Socié G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375–6382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources