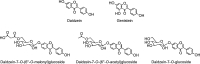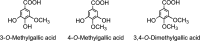Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases - PubMed (original) (raw)
Review
. 2013 May 10;18(14):1818-92.
doi: 10.1089/ars.2012.4581. Epub 2012 Aug 27.
Affiliations
- PMID: 22794138
- PMCID: PMC3619154
- DOI: 10.1089/ars.2012.4581
Review
Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases
Daniele Del Rio et al. Antioxid Redox Signal. 2013.
Abstract
Human intervention trials have provided evidence for protective effects of various (poly)phenol-rich foods against chronic disease, including cardiovascular disease, neurodegeneration, and cancer. While there are considerable data suggesting benefits of (poly)phenol intake, conclusions regarding their preventive potential remain unresolved due to several limitations in existing studies. Bioactivity investigations using cell lines have made an extensive use of both (poly)phenolic aglycones and sugar conjugates, these being the typical forms that exist in planta, at concentrations in the low-μM-to-mM range. However, after ingestion, dietary (poly)phenolics appear in the circulatory system not as the parent compounds, but as phase II metabolites, and their presence in plasma after dietary intake rarely exceeds nM concentrations. Substantial quantities of both the parent compounds and their metabolites pass to the colon where they are degraded by the action of the local microbiota, giving rise principally to small phenolic acid and aromatic catabolites that are absorbed into the circulatory system. This comprehensive review describes the different groups of compounds that have been reported to be involved in human nutrition, their fate in the body as they pass through the gastrointestinal tract and are absorbed into the circulatory system, the evidence of their impact on human chronic diseases, and the possible mechanisms of action through which (poly)phenol metabolites and catabolites may exert these protective actions. It is concluded that better performed in vivo intervention and in vitro mechanistic studies are needed to fully understand how these molecules interact with human physiological and pathological processes.
Figures
FIG. 1.
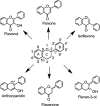
Structure of the flavonoid skeleton.
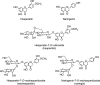
FIG. 2.
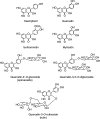
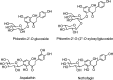
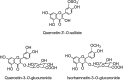
Structures of the flavonol aglycones kaempferol, quercetin, isorhamnetin, and myricetin and three common quercetin- O -glycosides.
FIG. 3.
The flavone aglycones apigenin, luteolin, baicalein, and wogonin; the flavone _C_-glycosides orientin, isoorientin, vitexin, and isovitexin; and the polymethoxylated flavones nobiletin and tangeretin.
FIG. 4.
The isoflavone aglycones daidzein and genistein and O -glycosides of daidzein, which along with similar genistein conjugates, are found in soy products.
FIG. 5.
Structures of plant lignans present in cereal grains and sesame seeds.
FIG. 6.
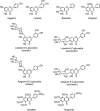
The S -enantiomers of the flavanone aglycones naringenin and hesperetin together with some of their naturally occurring glycosides.
FIG. 7.
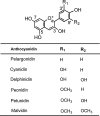
Structures of the major anthocyanidins.
FIG. 8.
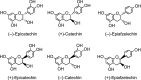
Structures of monomeric flavan-3-ol enantiomers.
FIG. 9.
Flavan-3-ol structures.
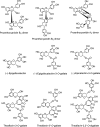
FIG. 10.
Naturally occurring dihydrochalcone glycosides.
FIG. 11.
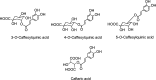
Gallic acid, ellagic acid, the gallotannin 2- O -digalloyl-tetra- O -galloyl-glucose, and the ellagitannins sanguiin H-6, and punicalagin.
FIG. 12.
The conjugated hydroxycinnamates 3- O -, 4- O -, and 5- O -caffeoylquinic acids and caftaric acid.
FIG. 13.
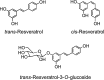
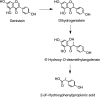
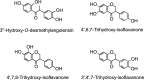
Common stilbene structures.
FIG. 14.
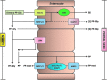
Proposed mechanisms for the absorption and metabolism of (poly)phenolic compounds in the small intestine. CBG, cytosolic β-glucosidase; COMT, catechol-_O_-methyl transferase; GLUT2, glucose transporter; LPH, lactase phloridzin hydrolase; MRP1-2–3, multidrug-resistant proteins; PP, (poly)phenol aglycone; PP-gly, (poly)phenol glycoside, PP-met, polyphenol sulfate/glucuronide/methyl metabolites; SGLT1, sodium-dependent glucose transporter; SULT, sulfotransferase; UGT, uridine-5′-diphosphate glucuronosyltransferase. (To see this illustration in color the reader is referred to the web version of this article at
.)
FIG. 15.
Structures of the quercetin metabolites quercetin-3′- _O_-sulfate, quercetin-3-_O_-glucuronide, and isorhamnetin-3-_O_-glucuronide (105, 321).
FIG. 16.
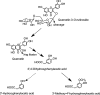
Proposed pathway for colonic bacterium-mediated catabolism of quercetin-3- O -rutinoside in the human large intestine resulting in the production of 3′,4′-dihydroxyphenylacetic acid and smaller quantities of 3′-hydroxyphenylacetic acid with subsequent hepatic conversion of 3′,4′-dihydroxyphenylacetic acid to 3′-methyoxy-4′-hydroxyphenylacetic acid before urinary excretion. Adapted from Jaganath et al. (220).
FIG. 17.
Narigenin and hesperetin metabolites (46, 49, 319).
FIG. 18.
Colonic phenolic acid catabolites of hesperetin.
FIG. 19.
Proposed pathways for the conversion of cyanidin-based red raspberry anthocyanins to phenolic acids and aromatic compounds. After consumption of 300 g of raspberries ∼40% of the ingested anthocyanins, principally cyanidin-_O_-glycosides, pass into the large intestine. When raspberry anthocyanins are incubated with fecal suspensions under anaerobic conditions, the cyanidin aglycone is released and catabolized by the colonic microflora undergoing C-ring fission, releasing phenolic acids, originating from both the A- and B-rings, which are metabolized via the pathways illustrated. Analysis of urine collected after raspberry consumption indicates that some of the colonic catabolites enter the circulatory and undergo further metabolism before being excreted in urine. These catabolites are thus detected in urine, but not fecal suspensions. F, catabolites detected in fecal suspensions; U, catabolites detected in urine (also highlighted in gray); *potential intermediates that did not accumulate in detectable quantities. Adapted from Gonzalez-Barrio et al. (159).
FIG. 20.
Daidzein metabolites detected in plasma and urine after acute supplementation with baked soy flour in milk. A similar array of genistein metabolites was also detected. Adapted from Hosoda, et al. (213).
FIG. 21.
Microbial metabolism of the isoflavonoid genistein. Adapted from Joannou et al. (225) and Setchell et al. (409).
FIG. 22.
Microbial metabolism of the isoflavonoid daidzein. Adapted from Coldham et al. (81) and Joannou et al. (225).
FIG. 23.
Human urinary metabolites excreted after the consumption of soy. Adapted from Heinonen et al. (186)
FIG. 24.
Human metabolism of the red clover isoflavones formononetin and biochanin A. Adapted from Heinonen et al. (185).
FIG. 25.
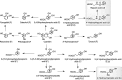
Proposed pathways involved in the colonic catabolism and urinary excretion of green tea flavan-3-ols. After consumption of green tea, more than 50% of the ingested flavan-3-ols (gray structures) pass into the large intestine. When incubated with fecal slurries, these compounds are catabolized by the colonic microflora, probably via the proposed pathways illustrated. Analysis of urine after green tea consumption indicates that some of the colonic catabolites enter the circulation and undergo further phase II metabolism before being excreted in urine. Structures in the gray box indicate such catabolites that are detected in urine, but not produced by fecal fermentation of (−)-epicatechin, (−)-epigallocatechin, or (−)-epigallocatechin-3-_O_-gallate. The dotted arrow between pyrogallol and pyrocatechol indicates that this is a minor conversion. Double arrows indicate conversions where the intermediates did not accumulate and are unknown. Compounds detected in ileal fluid after green tea consumption (IF); catabolites detected in fecal slurries (F) and in urine (U); potential intermediates that did not accumulate in detectable quantities in fecal slurries (*). Adapted from Roowi et al. (387).
FIG. 26.
Human (−)-epicatechin plasma metabolites detected in plasma after the consumption of an–(−)-epicatechin-rich cocoa drink. Adapted from Ottaviani et al. (337).
FIG. 27.
Methylated gallic acid derivatives excreted after the consumption of black tea.
FIG. 28.
Proposed pathways for human microbial degradation of procyanidin B1 dimer. Main routes are indicated with solid arrows, and minor pathways with dotted arrows. Metabolites derived from upper and lower units are grouped within the shaded rectangles. Adapted from Appeldoorn et al. (10) and Stouppi et al. (444, 445).
FIG. 29.
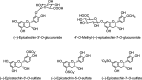
Metabolism of phloretin- O -glycosides after ingestion. Adapted from Richling (377).
FIG. 30.
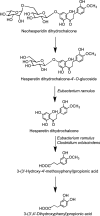
Catabolism of neohesperidin dihydrochalcone to 3-(3′,4′-dihydroxyphenyl)propionic acid by human fecal slurries. Steps catalyzed by the colonic bacteria Eubacterium ramulus and Clostridium orbiscindens are indicated. Adapted from Braune et al. (45).
FIG. 31.
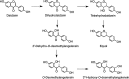
Potential pathways for the conversion of ellagitannins to urolithins.
FIG. 32.
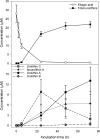
Anaerobic metabolism of punicalagins to ellagic acid and urolithins in fecal suspensions. Bars represent standard errors (n_=5). The initial concentration of punicalagin was 100 μ_M. Adapted from Gonzalez-Barrio et al. (159).
FIG. 33.
Anaerobic metabolism of ellagic acid to urolithins in fecal suspensions. Bars represent standard errors (n_=4). The initial concentration of ellagic acid was 50 μ_M. Adapted from Gonzalez-Barrio et al. (159).
FIG. 34.
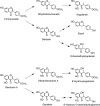
Proposed metabolism of chlorogenic acids following the ingestion of coffee by volunteers. 5-Caffeoylquinic acid and 5-feruloylquinic acid are illustrated structures, but their respective 3- and 4-isomers would be metabolized in a similar manner. Co-A, Co-enzyme A; EST, esterase; RA, reductase. Bold arrows indicate major routes, and dotted arrows minor pathways. Steps blocked in subjects with an ileostomy, and hence occurring in the colon are indicated. Adapted from Stalmach et al. (436, 439).
FIG. 35.
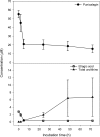
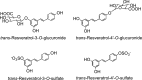
Human metabolites of trans -resveratrol. Adapted from Boocock et al. (40) and Patel et al. (348).
FIG. 36.
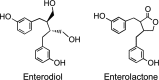
The mammalian lignans enterodiol and enterolactone.
FIG. 37.
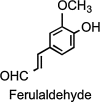
Ferulaldehyde.
FIG. 38.
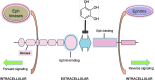
The Eph–ephrin system regulates cell adhesion, shape and movements, cell proliferation, survival, differentiation, and secretion and plays a role in embryogenesis, neural development, plasticity and regeneration, angiogenesis, bone remodeling, insulin secretion, intestinal homeostasis, cancer suppression, and/or promotion (347). Pyrogallol (1,2,3-trihydroxybenzene), a common colonic metabolite of several (poly)phenolic compounds, has been reported as able to perturb the protein–protein interactions in a dose-dependent way (457). (To see this illustration in color the reader is referred to the web version of this article at
.)
FIG. 39.
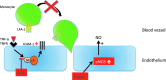
Graphical summary of some of the mechanisms involved in cardiovascular protection exerted by (poly)phenol metabolites. Protocatechuic acid (3,4-dihydroxybenzoic acid) impairs monocyte adhesion by inhibiting the nuclear content of p65, a subunit of NF-κB, and thus decreasing ICAM-1 and VCAM-1 expression. Moreover, equol induces eNOS expression by increasing NO production. eNOS, endothelial nitric oxide synthase; ICAM-1, intercellular adhesion molecule 1; LFA-1, lymphocyte function-associated antigen-1; NF-κB, nuclear factor kappa-light-chain enhancer of activated B cells; NO, nitric oxide; PCA, protocatechuic acid; TNF-α, tumor necrosis factor-α; TNFR, TNF-α receptor. (To see this illustration in color the reader is referred to the web version of this article at
.)
FIG. 40.
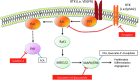
Graphical summary of some of the mechanisms involved in pathways related to cancer. MAPK/ERK signaling is inhibited by quercetin-3-_O_-glucuronide and enhanced by PCA and quercetin-3′-_O_-sulfate. JNK activity is reduced by urolithin A and increased by protocatechuic acid. Pyrogallol (1,2,3-trihydroxybenzene) inhibits EphA2 kinase activation, interfering with ephrinA1 binding (see also Fig. 39). Arrows identify activation interactions, whereas truncated lines classify inhibition interactions. Dotted lines are used to simplify transduction pathways. ERK, extracellular signal-regulated kinases; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; RTK, receptor tyrosine kinase; VEGFR, vascular endothelial growth factor receptor. (To see this illustration in color the reader is referred to the web version of this article at
.)
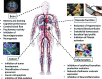
FIG. 41.
A summary of the potential health benefits of dietary (poly)phenols. (To see this illustration in color the reader is referred to the web version of this article at
.)
Similar articles
- Exploring and disentangling the production of potentially bioactive phenolic catabolites from dietary (poly)phenols, phenylalanine, tyrosine and catecholamines.
Clifford MN, Ludwig IA, Pereira-Caro G, Zeraik L, Borges G, Almutairi TM, Dobani S, Bresciani L, Mena P, Gill CIR, Crozier A. Clifford MN, et al. Redox Biol. 2024 May;71:103068. doi: 10.1016/j.redox.2024.103068. Epub 2024 Feb 13. Redox Biol. 2024. PMID: 38377790 Free PMC article. Review. - Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update.
Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D, Crozier A. Rodriguez-Mateos A, et al. Arch Toxicol. 2014 Oct;88(10):1803-53. doi: 10.1007/s00204-014-1330-7. Epub 2014 Sep 3. Arch Toxicol. 2014. PMID: 25182418 Review. - Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols.
Williamson G, Clifford MN. Williamson G, et al. Biochem Pharmacol. 2017 Sep 1;139:24-39. doi: 10.1016/j.bcp.2017.03.012. Epub 2017 Mar 18. Biochem Pharmacol. 2017. PMID: 28322745 Review. - Berry flavonoids and phenolics: bioavailability and evidence of protective effects.
Del Rio D, Borges G, Crozier A. Del Rio D, et al. Br J Nutr. 2010 Oct;104 Suppl 3:S67-90. doi: 10.1017/S0007114510003958. Br J Nutr. 2010. PMID: 20955651 Review. - Bioavailability of mango (poly)phenols: An evaluation of the impact of the colon, and phenylalanine and tyrosine on the production of phenolic catabolites.
Cáceres-Jiménez S, Pereira-Caro G, Dobani S, Pourshahidi K, Gill CIR, Moreno-Rojas JM, Ordoñez-Díaz JL, Almutairi TM, Clifford MN, Crozier A. Cáceres-Jiménez S, et al. Free Radic Biol Med. 2024 Oct 18;225:605-616. doi: 10.1016/j.freeradbiomed.2024.10.289. Online ahead of print. Free Radic Biol Med. 2024. PMID: 39426756
Cited by
- Metal-Phenolic Networks: A Promising Frontier in Cancer Theranostics.
Li L, Pan J, Huang M, Sun J, Wang C, Xu H. Li L, et al. Int J Nanomedicine. 2024 Nov 6;19:11379-11395. doi: 10.2147/IJN.S491421. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39524920 Free PMC article. Review. - Exploring the Potential of Anthocyanins for Repairing Photoaged Skin: A Comprehensive Review.
Guo X, He L, Sun J, Ye H, Yin C, Zhang W, Han H, Jin W. Guo X, et al. Foods. 2024 Nov 1;13(21):3506. doi: 10.3390/foods13213506. Foods. 2024. PMID: 39517290 Free PMC article. Review. - Free and bound phenolic profiles and antioxidant ability of eleven marine macroalgae from the South China Sea.
Peng Z, Wu Y, Fu Q, Xiao J. Peng Z, et al. Front Nutr. 2024 Oct 14;11:1459757. doi: 10.3389/fnut.2024.1459757. eCollection 2024. Front Nutr. 2024. PMID: 39469329 Free PMC article. - The association between dietary polyphenol intake and the odds of metabolic syndrome.
Makhtoomi M, Shateri Z, Mashoufi A, Nouri M, Honarvar B, Keshani P. Makhtoomi M, et al. Sci Rep. 2024 Oct 26;14(1):25559. doi: 10.1038/s41598-024-77335-4. Sci Rep. 2024. PMID: 39462087 Free PMC article. - The Potential Role of Polyphenol Supplementation in Preventing and Managing Depression: A Review of Current Research.
Farhan M, Faisal M. Farhan M, et al. Life (Basel). 2024 Oct 21;14(10):1342. doi: 10.3390/life14101342. Life (Basel). 2024. PMID: 39459643 Free PMC article. Review.
References
- Adlercreutz H. Lignans and human health. Crit Rev Clin Lab Sci. 2007;44:483–525. - PubMed
- Agewall S. Wright S. Doughty RN. Whalley GA. Duxbury M. Sharpe N. Does a glass of red wine improve endothelial function? Eur Heart J. 2000;21:74–78. - PubMed
- Aggarwal BB. Sung B. The relationship between inflammation and cancer is analogous to that between fuel and fire. Oncology. 2011;25:414–418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical