Creating a tumor-resistant microenvironment: cell-mediated delivery of TNFα completely prevents breast cancer tumor formation in vivo - PubMed (original) (raw)
. 2013 Feb 1;12(3):480-90.
doi: 10.4161/cc.23370. Epub 2012 Feb 1.
Ahmed F Salem, Ubaldo E Martinez-Outschoorn, Diana Whitaker-Menezes, Rebecca Lamb, James Hulit, Anthony Howell, Ricardo Gandara, Marina Sartini, Hwyda Arafat, Generoso Bevilacqua, Federica Sotgia, Michael P Lisanti
Affiliations
- PMID: 23292149
- PMCID: PMC3587449
- DOI: 10.4161/cc.23370
Creating a tumor-resistant microenvironment: cell-mediated delivery of TNFα completely prevents breast cancer tumor formation in vivo
Mazhar Al-Zoubi et al. Cell Cycle. 2013.
Abstract
Here, we provide the necessary proof of concept, that it is possible to metabolically create a non-permissive or "hostile" stromal microenvironment, which actively prevents tumor engraftment in vivo. We developed a novel genetically engineered fibroblast cell line that completely prevents tumor formation in mice, with a 100% protection rate. No host side effects were apparent. This could represent a viable cellular strategy for preventing and treating a variety of human cancers. More specifically, we examined the autocrine and paracrine effects of the cellular delivery of TNFα on breast cancer tumor growth and cancer metabolism. For this purpose, we recombinantly overexpressed TNFα in human breast cancer cells (MDA-MB-231) or human immortalized fibroblasts (hTERT-BJ1). Our results directly show that TNFα functions as a potent tumor suppressor. Remarkably, TNFα-expressing breast cancer cells were viable, without any significant increases in their basal apoptotic rate. However, after 4 weeks post-implantation, TNFα-expressing breast cancer cells failed to form any tumors in xenografted mice (0 tumors/10 injections), ultimately conferring 100% protection against tumorigenesis. Similarly, TNFα-overexpressing fibroblasts were also viable, without any increases in apoptosis. Significantly, complete tumor suppression was obtained by co-injecting TNFα expressing stromal fibroblasts with human breast cancer cells, indicating that paracrine cell-mediated delivery of TNFα can also prevent tumor engraftment and growth (0 tumors/10 injections). Mechanistically, TNFα induced autophagy and mitochondrial dysfunction in both epithelial cancer cells and stromal fibroblasts, preventing energy transfer from the tumor microenvironment, likely "starving" the cancer cells to death. In addition, via qRT-PCR analysis of MDA-MB-231 cells, we observed that TNFα mediated the upregulation of gene transcripts associated with inflammation and senescence [IL-1-β, IL-6, IL-8, MCP-1, COX-2, p21(WAF1/CIP1)] and downregulated known tumor-promoting genes (collagen VI and MMP2). Recombinant overexpression of TNFα receptor(s) in MDA-MB-231 cells also significantly reduced tumor growth, but was not as effective as the TNFα ligand itself in preventing tumor growth. Thus, we propose that stromal cell-mediated delivery of TNFα to human tumors [using transfected fibroblasts or mesenchymal stem cells (hMSCs)] may be a novel and effective strategy for the prevention and treatment of human cancers.
Keywords: apoptosis; autophagy; breast cancer; cancer prevention; cellular therapy; fibroblast mediated delivery; mitochondrial dysfunction; tumor cell engraftment; tumor growth; tumor necrosis factor (TNF).
Figures
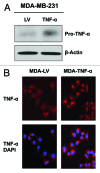
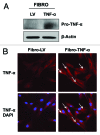
Figure 1. Stable TNFα overexpression in human epithelial breast cancer cells. MDA-MB-231 human breast cancer cells were transduced with a TNFα lentiviral vector or the LV empty vector control, and selected with puromycin to generate stable cell lines. Then, transduced cells were subjected to immunoblotting and immunofluorescence analysis to validate TNFα overexpression. (A) Immunoblotting shows a band at ~24 KDa, corresponding to the pro-TNFα precursor form. β-actin is shown as a control for equal protein loading. (B) Immunofluorescence with anti-TNFα antibodies demonstrates TNFα overexpression in cancer cells. Red, TNFα specific staining; blue, DAPI nuclear staining.
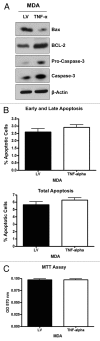
Figure 2. MDA-MB-231 cells overexpressing TNFα show the upregulation of apoptotic machinery, without a steady-state induction of increased apoptosis. (A) MDA-MB-231 cells overexpressing TNFα or the LV empty vector were subjected to immunoblotting with specific antibodies directed against apoptotic markers. Note that caspase-3 is significantly upregulated and activated in TNFα-expressing MDA-MB-231 cells, while Bax is downregulated consistent with BCL-2 upregulation. β-actin is shown as a control for equal loading. (B) Apoptosis was directly measured with Annexin-V and PI probes by flow cytometry (FACS analysis). MDA-MB-231 cells overexpressing TNFα did not show any significant increases in apoptotic rates relative to the empty vector control. Early and late apoptosis, represents Annexin-V-positive/PI-negative cells; Total apoptosis, represents Annexin-V-positive and/or PI-positive cells. (C) MTT assay demonstrates that MDA-MB-231 cells overexpressing TNFα show no differences in cell viability/proliferation, relative to empty vector control cells. These results indicate that TNFα does not increase the steady-state apoptotic rate in epithelial cancer cells.
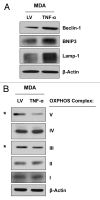
Figure 3. MDA-MB-231 cells overexpressing TNFα display the induction of autophagy and a reduction in mitochondrial OXPHOS. MDA-MB-231 cells overexpressing TNFα or LV empty vector were subjected to immunoblotting with specific antibodies directed against autophagic markers and OXPHOS complex components. (A) Note that several autophagy markers (Beclin-1, BNIP3 and Lamp-1) are upregulated in TNFα-overexpressing MDA-MB-231 cells relative to control cells. (B) MDA-MB-231 cells overexpressing TNFα display the downregulation of the OXPHOS complexes III and V, relative to control cells. β-actin is shown as an equal loading control.
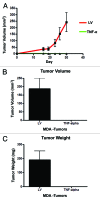
Figure 4. TNFα overexpression in cancer cells completely abolishes tumor growth in vivo. MDA-MB-231 cells (carrying TNFα or the LV empty vector control) were injected into the flanks of nude mice. Tumors growth was serially followed using calipers for 30 d post-injection. (A) Note that the growth curves clearly show that MDA-MB-231 cells overexpressing TNFα did not develop any tumors. After mice were sacrificed, tumor volume (B) and weight (C) were measured, demonstrating no growth of TNFα-MDA-MB-231 cells in all xenografted mice (0/10; 0 tumors grew from 10 injections). Thus, expression of TNFα confers 100% protection against tumor formation.
Figure 5. Stable TNFα overexpression in immortalized human fibroblasts. hTERT-BJ1 fibroblasts were transduced with a TNFα expressing vector or LV empty vector control. Then, the resulting transduced fibroblasts were subjected to immunoblotting or immunofluorescence analysis to validate TNFα overexpression. (A) Immunoblotting shows a band at ~24 KDa, corresponding to pro-TNFα, the precursor form. β-actin demonstrates equal protein loading. (B) Immunofluorescence with TNFα specific antibodies clearly demonstrates TNFα expression (see white arrows) in hTERT-BJ1 cells. Red, TNFα specific staining; blue, DAPI nuclear staining.
Figure 6. TNFα overexpression induces autophagy and deregulates mitochondrial activity in fibroblasts, without inducing apoptosis. (A) Immunoblotting shows that fibroblasts overexpressing TNFα have similar levels of the apoptotic markers (caspase-3, Bax and BCL-2), as control cells. (B) Apoptosis was measured with Annexin-V and PI probes by flow cytometry (FACS analysis). hTERT-BJ1 fibroblasts overexpressing TNFα did not show any significant increases in apoptosis, relative to empty vector controls. Early and late apoptosis, represents Annexin-V-positive/PI-negative cells; total apoptosis, represents Annexin-V-positive and/or PI-positive cells. (C) TNFα overexpression in hTERT-BJ1 cells induces autophagy, as assessed by the elevated expression and/or activation of cathepsin B, Lamp-1 and LC3B (LC3B-II is the cleaved active form). (D) Immunoblotting shows that TNFα overexpression in hTERT-BJ1 cells induces the downregulation of key subunits of the OXPHOS complex, namely components III and V. β-actin indicates equal protein loading.
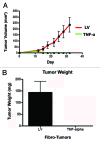
Figure 7. Fibroblasts harboring TNFα completely prevent breast cancer tumor growth in vivo. MDA-MB-231 cells were co-injected with hTERT-BJ1 cells (carrying TNFα or the LV empty vector control) into the flanks of nude mice. Tumors growth was serially measured using calipers for 31 d. (A) Note that the growth curves clearly show that MDA-MB-231 cells co-injected with TNFα-overexpressing fibroblasts did not develop any tumors. After 31 d, the mice were sacrificed. Measurements of tumor weight (B) show that TNFα-overexpressing fibroblasts completely prevented tumor growth of co-injected MDA-MB-231 cells, relative to control cells.
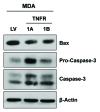
Figure 8. Overexpression of TNFα receptors induces the upregulation of apoptotic markers in cancer cells. MDA-MB-231 cells were transduced with TNFα receptor (TNFR-1A and TNFR-1B) vectors or an LV empty vector control and subjected to immunoblotting with apoptotic markers. Note the upregulation and activation of caspase-3 in MDA-MB-231 harboring TNFR-1A and TNFR-1B, relative to empty vector control cells. β-actin is shown as a control for equal protein loading.
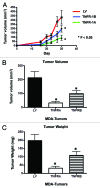
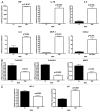
Figure 9. Cancer cells overexpressing TNFR-1A or TNFR-1B show significant tumor growth inhibition in vivo. MDA-MB-231 cells (carrying TNFR-1A, TNFR-1B or the LV empty vector control) were injected into the flanks of nude mice. Tumors were measured with calipers for 30 d. Note that the serial growth curve (A), final tumor volume (B) and final tumor weight (C) all clearly show that TNFR-1A and TNFR-1B transduced MDA-MB-231 cells display significantly reduced tumor growth relative to control cells.
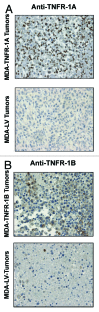
Figure 10. TNFR-1A and TNFR-1B overexpression in MDA-MB-231 cell tumor xenografts. TNFR-1A and TNFR-1B overexpression in MDA-MB-231 cells was validated on the tumor xenografts derived from TNFR-1A, TNFR-1B or LV-control MDA-MB-231 cells. Sections were subjected to immunohistochemistry with anti-TNFR-1A (A) or anti-TNFR-1B (B) antibodies. The positive-staining (brown color) represents TNFR-1A or TNFR-1B overexpression. Original magnification, 60×.
Figure 11. MDA-MB-231 cancer cells overexpressing TNFα display elevated transcripts associated with inflammation and senescence and decreased transcripts associated with extracellular matrix remodeling. MDA-MB-231 cells overexpressing TNFα or vector alone were analyzed by quantitative real time PCR (qRT-PCR) for markers of inflammation, senescence and extracellular matrix remodeling. (A) Note that TNFα induces the upregulation of several transcripts associated with an inflammatory response, including IL-1-β, IL-8, IL-6, MCP-1 and COX-2. TNFα was also found to be elevated, as expected. (B) In addition, several markers associated with extracellular matrix remodeling, including collagen VI and MMP2, were selectively downregulated in TNFα-expressing cancer cells, relative to controls. (C) Finally, a marker of hypoxia, HIF2a, was decreased and a marker of senescence, p21 (Cip1/Waf1) was found to be elevated in TNFα-expressing cancer cells, relative to controls. We have previously shown that increased HIF2a expression in MDA-MB-231 cancer cells promotes tumor growth, and that increased p21 expression in MDA-MB-231 cancer cells inhibits tumor growth.,
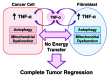
Figure 12. Proposed metabolic mechanism(s) underlying the potent tumor suppressor effects of TNFα in the tumor microenvironment. TNFα can be release either by tumor cells or cancer associated fibroblasts (CAFs). Then, TNFα induces autophagy both in the fibroblast cell compartment and in the epithelial cancer cell compartment. In addition, TNFα induces mitochondrial dysfunction in both compartents. The resulting lack of energy transfer between the two metabolic comparments (epithelia vs. stroma) promotes complete tumor regression and/or inhibits tumor growth.
Similar articles
- Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment.
Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, Wang C, Pavlides S, Martinez-Cantarin MP, Capozza F, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Caro J, Lisanti MP, Sotgia F. Martinez-Outschoorn UE, et al. Cell Cycle. 2010 Sep 1;9(17):3515-33. doi: 10.4161/cc.9.17.12928. Epub 2010 Sep 9. Cell Cycle. 2010. PMID: 20855962 Free PMC article. - CTGF drives autophagy, glycolysis and senescence in cancer-associated fibroblasts via HIF1 activation, metabolically promoting tumor growth.
Capparelli C, Whitaker-Menezes D, Guido C, Balliet R, Pestell TG, Howell A, Sneddon S, Pestell RG, Martinez-Outschoorn U, Lisanti MP, Sotgia F. Capparelli C, et al. Cell Cycle. 2012 Jun 15;11(12):2272-84. doi: 10.4161/cc.20717. Epub 2012 Jun 15. Cell Cycle. 2012. PMID: 22684333 Free PMC article. - Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: connecting TGF-β signaling with "Warburg-like" cancer metabolism and L-lactate production.
Guido C, Whitaker-Menezes D, Capparelli C, Balliet R, Lin Z, Pestell RG, Howell A, Aquila S, Andò S, Martinez-Outschoorn U, Sotgia F, Lisanti MP. Guido C, et al. Cell Cycle. 2012 Aug 15;11(16):3019-35. doi: 10.4161/cc.21384. Epub 2012 Aug 9. Cell Cycle. 2012. PMID: 22874531 Free PMC article. - Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function.
Martinez-Outschoorn U, Sotgia F, Lisanti MP. Martinez-Outschoorn U, et al. Semin Oncol. 2014 Apr;41(2):195-216. doi: 10.1053/j.seminoncol.2014.03.002. Epub 2014 Mar 5. Semin Oncol. 2014. PMID: 24787293 Review. - Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment.
Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Tanowitz HB, Sotgia F, Lisanti MP. Martinez-Outschoorn UE, et al. Int J Biochem Cell Biol. 2011 Jul;43(7):1045-51. doi: 10.1016/j.biocel.2011.01.023. Epub 2011 Feb 15. Int J Biochem Cell Biol. 2011. PMID: 21300172 Free PMC article. Review.
Cited by
- Osteopontin (OPN) isoforms, diabetes, obesity, and cancer; what is one got to do with the other? A new role for OPN.
Sarosiek K, Jones E, Chipitsyna G, Al-Zoubi M, Kang C, Saxena S, Gandhi AV, Sendiky J, Yeo CJ, Arafat HA. Sarosiek K, et al. J Gastrointest Surg. 2015 Apr;19(4):639-50. doi: 10.1007/s11605-014-2735-6. Epub 2015 Jan 13. J Gastrointest Surg. 2015. PMID: 25583441 - Overexpressing TNF-alpha in pancreatic ductal adenocarcinoma cells and fibroblasts modifies cell survival and reduces fatty acid synthesis via downregulation of sterol regulatory element binding protein-1 and activation of acetyl CoA carboxylase.
Al-Zoubi M, Chipitsyna G, Saxena S, Sarosiek K, Gandhi A, Kang CY, Relles D, Andrelsendecki J, Hyslop T, Yeo CJ, Arafat HA. Al-Zoubi M, et al. J Gastrointest Surg. 2014 Feb;18(2):257-68; discussion 268. doi: 10.1007/s11605-013-2370-7. Epub 2013 Oct 4. J Gastrointest Surg. 2014. PMID: 24091913 - The roles of mesenchymal stem cells in tumor inflammatory microenvironment.
Sun Z, Wang S, Zhao RC. Sun Z, et al. J Hematol Oncol. 2014 Feb 6;7:14. doi: 10.1186/1756-8722-7-14. J Hematol Oncol. 2014. PMID: 24502410 Free PMC article. Review. - Oncodriver inhibition and CD4+ Th1 cytokines cooperate through Stat1 activation to induce tumor senescence and apoptosis in HER2+ and triple negative breast cancer: implications for combining immune and targeted therapies.
Rosemblit C, Datta J, Lowenfeld L, Xu S, Basu A, Kodumudi K, Wiener D, Czerniecki BJ. Rosemblit C, et al. Oncotarget. 2018 May 1;9(33):23058-23077. doi: 10.18632/oncotarget.25208. eCollection 2018 May 1. Oncotarget. 2018. PMID: 29796172 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous