A variant on the kappa opioid receptor gene (OPRK1) is associated with stress response and related drug craving, limbic brain activation and cocaine relapse risk - PubMed (original) (raw)
A variant on the kappa opioid receptor gene (OPRK1) is associated with stress response and related drug craving, limbic brain activation and cocaine relapse risk
K Xu et al. Transl Psychiatry. 2013.
Abstract
Stress increases drug craving and relapse risk. The kappa opioid receptor gene (OPRK1) mediates stress responses. Here, we examined whether the OPRK1 rs6989250 C>G affects stress-induced cocaine craving and cortisol responses, subsequent cocaine relapse risk and the neural response to stress using functional magnetic resonance imaging (fMRI) in cocaine dependence. Sixty-seven treatment-engaged, abstinent cocaine-dependent African-Americans were genotyped (CG: N=10; CC: N=57) and participated in a 3-day experiment in which they were exposed to personalized script-driven imagery of stress, drug cues and neutral scenarios, one condition per day, randomly assigned and counterbalanced across subjects. Repeated measures of craving and cortisol were obtained. The subjects were followed prospectively for 90 days to assess relapse risk. A follow-up preliminary fMRI experiment assessed neural responses to stress, drug cue and neutral conditions in matched CG (N=5) and CC (N=8) subgroups. We found greater stress-induced craving (P=0.019), higher cortisol during stress and cue relative to the neutral condition (P's<0.003), and increased cocaine relapse risk (P=0.0075) in the CG compared with the CC group. The CG relative to the CC group also showed greater activation of limbic and midbrain regions during stress and cues relative to the neutral condition with additional stress-induced activation in the right amygdala/hippocampus (P<0.05, whole-brain corrected). These results suggest that OPRK1 is associated with stress-induced craving and cortisol, hyperactive hypothalamus/thalamus-midbrain-cerebellum responses, and also associated with greater subsequent cocaine relapse risk. Future studies to replicate these findings in a larger sample size are warranted.
Figures
Figure 1
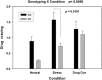
Mean (and standard error) drug craving response (averaged across all time points) during each of the neutral (N), stress (S) and drug cue (T) conditions for the two OPRK1 rs6989250 genotypes is shown. The genotype-by-condition two-way interaction was significant (_P_=0.0056, Cohen's _d_=0.79). Carriers of the CG genotype showed markedly stronger craving when exposed to the stress condition compared with individuals with the CC genotype (_P_=0.0191, Cohen's _d_=0.82). There was no significant difference detected between the two genotype groups in either the neutral (_P_=0.0823) or drug cue conditions (_P_=0.678).
Figure 2
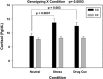
Mean (and standard error) plasma cortisol levels averaged across all time points in the neutral (N), stress (S) and drug cue (T) conditions for the OPRK1 rs6989250 genotype groups are shown. There was a significant genotype–condition interaction (_P_=0.0003, Cohen's _d_=1.01), with CG individuals showing higher cortisol levels during stress compared with the neutral and drug cue conditions (P<0.0001 for stress versus neutral Cohen's _d_=1.46; _P_=0.003, Cohen's _d_=1.04 for cue versus neutral), whereas CC individuals showed no stress-induced increases in cortisol compared with their cortisol responses in the drug cue and neutral conditions (_P_'s>0.05).
Figure 3
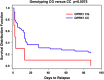
Log-rank survival analysis for abstinence versus cocaine relapse in the CG and CC genotype groups. The survival rate for the CG genotype group was significantly lower than for the CC genotype group (Log-rank _χ_2=5.242; _P_=0.0075). Within 14 days, only 10% individuals with the CG genotype remain abstinence of cocaine use; 45% individuals with the CC genotype remain abstinence. The Y axis shows the proportion of participants who did not relapse; the X axis shows the 90-day follow-up time period.
Figure 4
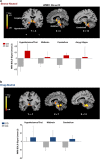
Whole brain analysis of neural activation during (a) stress and (b) drug cue exposure in the CG group relative to the CC group. During the stress and drug cue relative to the neutral condition, the CG group showed increased activity in the hypothalamus, midbrain (ventral tegmental area and locus coeruleus), thalamus and cerebellum relative to the CC group. Additionally, the CG group displayed increased stress-induced activity in the right amygdala/hippocampus and periaqueductal gray (PAG) area of the midbrain compared with the CC group (P<0.05, whole-brain family-wise error (FWE) corrected). The blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) signal in each significant cluster (Table 2) is displayed in bar graphs for each genotype group in (a) stress-neutral and (b) drug-neutral conditions (see Table 2 for _t_-values and Cohen's d). Coordinates are given in Montreal Neurological Institute (MNI) space.
Similar articles
- The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type.
Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R. Fox HC, et al. Psychoneuroendocrinology. 2013 Sep;38(9):1532-44. doi: 10.1016/j.psyneuen.2012.12.022. Epub 2013 Jan 29. Psychoneuroendocrinology. 2013. PMID: 23374328 Free PMC article. Clinical Trial. - Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence.
Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Potenza MN, et al. Am J Psychiatry. 2012 Apr;169(4):406-14. doi: 10.1176/appi.ajp.2011.11020289. Am J Psychiatry. 2012. PMID: 22294257 Free PMC article. - Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues.
Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Fox HC, et al. Psychoneuroendocrinology. 2005 Oct;30(9):880-91. doi: 10.1016/j.psyneuen.2005.05.002. Psychoneuroendocrinology. 2005. PMID: 15975729 Clinical Trial. - Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors.
Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Weiss F, et al. Ann N Y Acad Sci. 2001 Jun;937:1-26. doi: 10.1111/j.1749-6632.2001.tb03556.x. Ann N Y Acad Sci. 2001. PMID: 11458532 Review. - Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications.
Sinha R, Li CS. Sinha R, et al. Drug Alcohol Rev. 2007 Jan;26(1):25-31. doi: 10.1080/09595230601036960. Drug Alcohol Rev. 2007. PMID: 17364833 Review.
Cited by
- Genetic Moderation of Stress Effects on Corticolimbic Circuitry.
Bogdan R, Pagliaccio D, Baranger DA, Hariri AR. Bogdan R, et al. Neuropsychopharmacology. 2016 Jan;41(1):275-96. doi: 10.1038/npp.2015.216. Epub 2015 Jul 20. Neuropsychopharmacology. 2016. PMID: 26189450 Free PMC article. Review. - Systemic kappa opioid receptor antagonism accelerates reinforcement learning via augmentation of novelty processing in male mice.
Farahbakhsh ZZ, Song K, Branthwaite HE, Erickson KR, Mukerjee S, Nolan SO, Siciliano CA. Farahbakhsh ZZ, et al. Neuropsychopharmacology. 2023 May;48(6):857-868. doi: 10.1038/s41386-023-01547-x. Epub 2023 Feb 17. Neuropsychopharmacology. 2023. PMID: 36804487 Free PMC article. - Variations in opioid receptor genes in neonatal abstinence syndrome.
Wachman EM, Hayes MJ, Sherva R, Brown MS, Davis JM, Farrer LA, Nielsen DA. Wachman EM, et al. Drug Alcohol Depend. 2015 Oct 1;155:253-9. doi: 10.1016/j.drugalcdep.2015.07.001. Epub 2015 Jul 8. Drug Alcohol Depend. 2015. PMID: 26233486 Free PMC article. - Pharmacogenetics of Addiction Therapy.
Graham DP, Harding MJ, Nielsen DA. Graham DP, et al. Methods Mol Biol. 2022;2547:437-490. doi: 10.1007/978-1-0716-2573-6_16. Methods Mol Biol. 2022. PMID: 36068473 - Anti-stress neuropharmacological mechanisms and targets for addiction treatment: A translational framework.
Greenwald MK. Greenwald MK. Neurobiol Stress. 2018 Aug 11;9:84-104. doi: 10.1016/j.ynstr.2018.08.003. eCollection 2018 Nov. Neurobiol Stress. 2018. PMID: 30238023 Free PMC article. Review.
References
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical