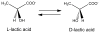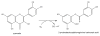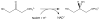Nickel-dependent metalloenzymes - PubMed (original) (raw)
Review
Nickel-dependent metalloenzymes
Jodi L Boer et al. Arch Biochem Biophys. 2014.
Abstract
This review describes the functions, structures, and mechanisms of nine nickel-containing enzymes: glyoxalase I, acireductone dioxygenase, urease, superoxide dismutase, [NiFe]-hydrogenase, carbon monoxide dehydrogenase, acetyl-coenzyme A synthase/decarbonylase, methyl-coenzyme M reductase, and lactate racemase. These enzymes catalyze their various chemistries by using metallocenters of diverse structures, including mononuclear nickel, dinuclear nickel, nickel-iron heterodinuclear sites, more complex nickel-containing clusters, and nickel-tetrapyrroles. Selected other enzymes are active with nickel, but the physiological relevance of this metal specificity is unclear. Additional nickel-containing proteins of undefined function have been identified.
Keywords: Catalytic mechanism; Enzyme; Metallocenter; Nickel; Protein structure.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Fig. 1
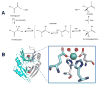
Glyoxalase I (GlxI). (A) GlxI acts on the reversibly-formed hemithioacetal product of glutathione (GSH) plus methylglyoxal, and catalyzes formation of _S_-D-lactoylglutathione via a _cis_-enediolate intermediate. The product is hydrolyzed by glyoxalase II (GlxII) to yield D-lactate. (B) Glx I structure and active site. The homodimeric protein (cyan and white cartoon view, PDB access code 1f9z, Escherichia coli) contains two Ni-containing active sites (green spheres) at the dimer interface with His and Glu ligands (sticks) contributed by each subunit and two coordinated waters (red spheres). (2 column width)
Fig. 2
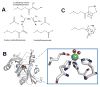
Acireductone dioxygenase (ARD). (A) The Fe-containing enzyme (left pathway) converts the substrate to formic acid and the keto-acid of Met, while the Ni-bound enzyme (right path) produces formic acid, CO, and 3-methylthiopropionate. (B) ARD structure and active site. The NMR structure of acireductone dioxygenase (white cartoon view, PDB access code 1zrr, Klebsiella oxytoca) is shown. The 6-coordinate Ni (green sphere) coordinates three His and one Glu (sticks), and two water molecules (red spheres). (C) Postulated reaction intermediates in the Fe- versus Ni-catalyzed reactions of ARD. Dioxygen and 1,2-dihydroxy-3-keto-5-methylthiopentene are thought to react at the ARD active site to form the metal-dependent cyclic peroxides shown. These rearrange, as shown by the arrows, to yield the appropriate products. (2 column width)
Fig. 3
Urease. (A) The reaction catalyzed by urease. (B) Urease structure and active site. The detailed architectures differ for different ureases, but all include a trimeric configuration of 1, 2, or 3 subunits. The urease shown (cartoon view, PDB access code 1fwj, Klebsiella aerogenes) possesses three subunits in uniform color (cyan, yellow, or white). Each active site contains two Ni (green spheres) that are bridged by a Lys carbamate and a water, with each Ni also binding a terminal water and two His, plus an Asp coordinating to one metal (side chains as sticks and solvent as red spheres). (C) Four reaction mechanisms proposed for urease. Proposals for urease catalysis are unified by having the urea carbonyl oxygen coordinated to Ni-1. (i) Hydroxide bound to Ni-2 attacks the urea carbonyl carbon to form a tetrahedral intermediate that decomposes with a nearby His residue functioning as a general acid. (ii) The bridging hydroxide attacks the urea carbonyl carbon while transferring its proton to product ammonia, with the other urea amine coordinated to Ni-2. (iii) The bridging hydroxide attacks the urea carbonyl carbon to form a tetrahedral intermediate that decomposes with a nearby His residue functioning as a general acid. (iv) A general base (perhaps Ni-2 coordinated hydroxide as shown) abstracts a urea proton leading to elimination of ammonia and production of cyanate that is subsequently hydrated. (2 column width)
Fig. 4
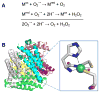
Superoxide dismutase (SOD). (A) The reaction catalyzed by SOD. (B) NiSOD structure and active site. The homohexameric structure of NiSOD (cartoon view, PDB access code 1t6u, Streptomyces coelicolor) is shown with each subunit a different color. One active site is shown in the oxidized form, with Ni3+ (green sphere) coordinated via the amino terminal amine, a backbone amide, two Cys, and an axial His (stick view). The His is displaced as a ligand for the reduced state (indicated by the dashed line). (1 column width)
Fig. 5
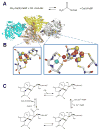
Hydrogenase. (A) The reaction catalyzed by hydrogenase. (B) Hydrogenase structure and active site. The dimeric [NiFe]-hydrogenase (cartoon view, PDB access code 1yrq, Desulfovibrio fructosovorans) contains three [FeS] clusters (brown and yellow spheres) in the small (cyan) subunit and the [NiFe] cluster in the large (white) subunit. An expanded view of the [NiFe] cluster is shown in stick view with the Ni (green sphere) coordinated by four Cys, two of which also coordinate Fe (brown sphere). In this state, a μ-oxo group (red sphere) bridges the two metals, and the Fe possesses a CO and two cyanide ligands. (C) Subset of the [NiFe] cluster states proposed for the [NiFe]-hydrogenase from Desulfovibrio vulgaris str. Miyazaki F. L1, L2, and L3 are diatomic ligands of Fe. (2 column width)
Fig. 6
CO dehydrogenase (CODH). (A) The reaction catalyzed by CODH. (B) The CODH structure and active site. Dimeric CODH (cartoon view, 3b53, Carboxydothermus hydrogenoformans) contains two [1Ni-4Fe-4S] clusters (green, brown, and yellow spheres) and three [4Fe-4S] clusters (brown and yellow spheres) including one which bridges the subunits. An expanded view of the [1Ni-4Fe-4S] cluster is shown with the ligands shown in stick view. (C) Mechanism of CODH. One proposal for the mechanism of CODH includes a Ni2+-hydride intermediate and retains Ni2+ in all steps; an alternative model invokes a Ni0 state following CO2 release. (2 column width)
Fig. 7
Acetyl-coenzyme A (acetyl-S-CoA) synthase/decarbonylase (ACS). (A) The reaction catalyzed by ACS. (B) ACS structure and active sites. ACS (cartoon view, PDB access code 2z8y, Morella thermoacetica) contains a CODH homodimer (yellow and sand) with two [1Ni-4Fe-4S] clusters (side chains shown in stick view, bottom left) and three [4Fe-4S] clusters, including one bridging the subunits, along with two subunits (cyan and gray cartoon) each containing a [Ni-M-4Fe-4S] cluster (side chains shown in stick view, bottom right). The structure shown is for an inactive protein where M is Cu (purple sphere), but the active enzyme possesses Ni at this site. (C) ACS mechanism. In this oversimplified view of the ACS mechanism, the Ni located distal to the [4Fe-4S] cluster retains its divalent state throughout catalysis whereas the proximal Ni cycles between the Ni2+ and Ni1+ states. Alternative mechanisms invoke Ni3+ or Ni0 states for the proximal metal site. (2 column width)
Fig. 8
Methyl-coenzyme M reductase (MCR). (A) The reaction catalyzed by MCR. (B) MCR structure and active site. MCR is a dimer of the αβγ subunits (cartoon view, PDB access code 1mro, Methanothermobacter marburgensis). Each active site (Ni as a green sphere with ligands in stick view) contains coenzyme F430 with an axial Gln ligand, and an additional axially coordinated CoM-SH in this particular structure. A line drawing of coenzyme F430 is illustrated for clarity, where R is H or S-CH3 depending on the source. (C) Two postulated reaction mechanism of MCR. In the upper pathway, the Ni1+ attacks the methyl group of methyl-S-CoM to form a methyl-Ni3+ intermediate. In the lower pathway, a CoM-S-Ni2+ intermediate is formed. Both pathways incorporate a disulfide anion radical. (2 column width)
Fig. 9
Reaction catalyzed by lactate racemase. (1 column width)
Fig. 10
Reaction catalyzed by the QueD quercetinase. (1.5 column width)
Fig. 11
Reaction catalyzed by AraM, a glycerol phosphate dehydrogenase. (1.5 column width)
Similar articles
- Structure, function, and biosynthesis of nickel-dependent enzymes.
Alfano M, Cavazza C. Alfano M, et al. Protein Sci. 2020 May;29(5):1071-1089. doi: 10.1002/pro.3836. Epub 2020 Feb 18. Protein Sci. 2020. PMID: 32022353 Free PMC article. Review. - Nickel-binding proteins.
Wattt RK, Ludden PW. Wattt RK, et al. Cell Mol Life Sci. 1999 Nov 15;56(7-8):604-25. doi: 10.1007/s000180050456. Cell Mol Life Sci. 1999. PMID: 11212309 Free PMC article. Review. - Surprising cofactors in metalloenzymes.
Drennan CL, Peters JW. Drennan CL, et al. Curr Opin Struct Biol. 2003 Apr;13(2):220-6. doi: 10.1016/s0959-440x(03)00038-1. Curr Opin Struct Biol. 2003. PMID: 12727516 Review. - Nickel enzymes in microbes.
Hausinger RP. Hausinger RP. Sci Total Environ. 1994 Jun 6;148(2-3):157-66. doi: 10.1016/0048-9697(94)90392-1. Sci Total Environ. 1994. PMID: 8029691 - Nickel uptake and utilization by microorganisms.
Mulrooney SB, Hausinger RP. Mulrooney SB, et al. FEMS Microbiol Rev. 2003 Jun;27(2-3):239-61. doi: 10.1016/S0168-6445(03)00042-1. FEMS Microbiol Rev. 2003. PMID: 12829270 Review.
Cited by
- Organoselenium transition metal complexes as promising candidates in medicine area.
Kostić M, Marjanović J, Divac V. Kostić M, et al. J Biol Inorg Chem. 2024 Sep;29(6):555-571. doi: 10.1007/s00775-024-02072-y. Epub 2024 Aug 9. J Biol Inorg Chem. 2024. PMID: 39123093 Review. - Targeting bacterial nickel transport with aspergillomarasmine A suppresses virulence-associated Ni-dependent enzymes.
Sychantha D, Chen X, Koteva K, Prehna G, Wright GD. Sychantha D, et al. Nat Commun. 2024 May 13;15(1):4036. doi: 10.1038/s41467-024-48232-1. Nat Commun. 2024. PMID: 38740750 Free PMC article. - Current status of carbon monoxide dehydrogenases (CODH) and their potential for electrochemical applications.
Bährle R, Böhnke S, Englhard J, Bachmann J, Perner M. Bährle R, et al. Bioresour Bioprocess. 2023 Nov 27;10(1):84. doi: 10.1186/s40643-023-00705-9. Bioresour Bioprocess. 2023. PMID: 38647803 Free PMC article. Review. - Overview of Proteus mirabilis pathogenicity and virulence. Insights into the role of metals.
Chakkour M, Hammoud Z, Farhat S, El Roz A, Ezzeddine Z, Ghssein G. Chakkour M, et al. Front Microbiol. 2024 Apr 5;15:1383618. doi: 10.3389/fmicb.2024.1383618. eCollection 2024. Front Microbiol. 2024. PMID: 38646633 Free PMC article. Review. - Proteus mirabilis UreR coordinates cellular functions required for urease activity.
Fitzgerald MJ, Pearson MM, Mobley HLT. Fitzgerald MJ, et al. J Bacteriol. 2024 Apr 18;206(4):e0003124. doi: 10.1128/jb.00031-24. Epub 2024 Mar 27. J Bacteriol. 2024. PMID: 38534115 Free PMC article.
References
- Mulrooney SB, Hausinger RP. FEMS Microbiol Rev. 2003;27:239–261. - PubMed
- Kaluarachchi H, Chan Chung KC, Zamble DB. Natural Product Reports. 2010;27:681–694. - PubMed
- Li Y, Zamble DB. Chem Rev. 2009;109:4617–4643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources