Granzyme M targets topoisomerase II alpha to trigger cell cycle arrest and caspase-dependent apoptosis - PubMed (original) (raw)
Granzyme M targets topoisomerase II alpha to trigger cell cycle arrest and caspase-dependent apoptosis
S A H de Poot et al. Cell Death Differ. 2014 Mar.
Abstract
Cytotoxic lymphocyte protease granzyme M (GrM) is a potent inducer of tumor cell death. The apoptotic phenotype and mechanism by which it induces cell death, however, remain poorly understood and controversial. Here, we show that GrM-induced cell death was largely caspase-dependent with various hallmarks of classical apoptosis, coinciding with caspase-independent G2/M cell cycle arrest. Using positional proteomics in human tumor cells, we identified the nuclear enzyme topoisomerase II alpha (topoIIα) as a physiological substrate of GrM. Cleavage of topoIIα by GrM at Leu(1280) separated topoIIα functional domains from the nuclear localization signals, leading to nuclear exit of topoIIα catalytic activity, thereby rendering it nonfunctional. Similar to the apoptotic phenotype of GrM, topoIIα depletion in tumor cells led to cell cycle arrest in G2/M, mitochondrial perturbations, caspase activation, and apoptosis. We conclude that cytotoxic lymphocyte protease GrM targets topoIIα to trigger cell cycle arrest and caspase-dependent apoptosis.
Figures
Figure 1
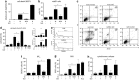
Cell death induced by GrM is characterized by apoptotic hallmarks. (a) HeLa cells were treated with 1 _μ_M GrM (M), 1 _μ_M GrM-SA (MSA), or 100 nM GrB, and 0.5 _μ_g/ml SLO or no SLO for 20 h at 37 °C. Metabolically active cells were quantified using the WST-1 assay, and the relative loss of viability compared with untreated (SLO-negative) cells was calculated. (b) Jurkat cells were treated with 1 _μ_M GrM (M), 1 _μ_M GrM-SA (MSA), or 100 nM GrB in the absence or presence of 0.1 μ_g/ml SLO for 16 h. DNA fragmentation was determined by PI staining and flow cytometry to assess the percentage of subG1 cells. (c) HeLa cells were treated as in panel (a) and tested for induction of apoptosis with AnnV/PI flow cytometry. Results are representative of at least three experiments. (d) HeLa cells were treated as in panel (a) for 4, 8, or 20 h and tested for induction of apoptosis with AnnV/PI flow cytometry. AnnV and/or PI-positive cells are plotted on the y axis. (e–h) HeLa cells were treated as in panel (a) and tested for (e) DNA fragmentation with TUNEL (results are representative of at least three experiments), (f) loss of mitochondrial membrane potential (Δ_Ψ m) with DiOC6, (g) generation of ROS with CM-H2DCFDA, and (h) release of cytochrome c using flow cytometry. Bar graphs depict the mean±S.D., with *P values<0.05
Figure 2
GrM indirectly activates caspases-3, -6, -8, and -9 and induces caspase-8-dependent and -independent cell death. (a) 5 _μ_g of HeLa cell lysate was incubated with the indicated concentrations of GrM (0–500 nM) or 500 nM GrB for 2 h at 37 °C in the absence (dimethyl sulfoxide (DMSO)) or presence of 100 _μ_M z-VAD-fmk, after which caspase activation was assessed using immunoblot. The effects of GrM on known substrates TRAP, NPM,_α_-tubulin, and FADD were also determined. Nm23H1 served as loading control. (b) HeLa cells were treated with 1 _μ_M GrM (M), 1 _μ_M GrM-SA (MSA), or 100 nM GrB (B) and 0.5 _μ_g/ml SLO in the absence (DMSO) or presence of 100 _μ_M z-VAD-fmk for 8 h at 37 °C. Caspase activation and substrate cleavage were visualized using immunoblot with nm23H1 serving as the loading control. Known GrM substrates (NPM, _α_-tubulin, and FADD) were used as positive controls, of which _α_-tubulin cleavage was partially mediated by caspases. (c) Jurkat cells were treated for 6 h at 37 °C with 1 _μ_M GrM, 1 _μ_M GrM-SA, or 50 nM GrB and 0.1 _μ_g/ml SLO. Caspase-3/-7 activation was detected using the CaspaseGlo assay. Relative light units (RLU) are depicted. (d–f) HeLa cells were treated with 1 _μ_M GrM or 1 _μ_M GrM-SA and 0.5 μ_g/ml SLO. Caspase activation (d) was detected in time using immunoblot analysis, and the kinetics of Δ_Ψ m (DiOC6) loss (e) and cytochrome c release (f) were also determined at various time intervals (_n_=3, means±S.E.M.). (g) HeLa cells were treated as in panel (b), and the effects of 100 _μ_M zVAD on GrM-induced cell death were determined using AnnV/PI flow cytometry. (h) Wild-type (wt) and caspase-8-deficient (casp-8−/−) Jurkat cells were treated with 1 _μ_M GrM and 0.1 _μ_g/ml SLO in the absence (DMSO) or presence of 100 _μ_M z-VAD-fmk. Cell death induction was assessed using AnnV/PI flow cytometry
Figure 3
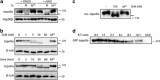
Positional proteomics-based identification of GrM-mediated topoII_α_ cleavage at Leu1280 in HeLa cells. (a) Schematic overview of the N-terminal positional proteomics strategy. L-Arg SILAC-labeled HeLa cells were incubated with 1 _μ_M GrM and 0.5 μ_g/ml SLO for either 15 (12C6) or 60 (13C6) min or with 0.5 μ_g/ml SLO only for 60 min (13C615N4) at 37 °C. N-terminal COFRADIC was used to identify in vivo GrM substrates. (b) IceLogo visualization of the 38 non-redundant P10-P10' GrM cleavage site motifs identified. Multiple sequence alignments of peptide substrate motifs from P10 to P10' are given with cleavage of the substrate occurring between P1 and P1'. Statistically significant residues with a P_-value threshold of≤0.05 are plotted. The amino-acid heights are indicative of their degree of conservation at the indicated position. The frequency of the amino-acid occurrence at each position in the sequence set was compared with the human Swissprot 2011_11 database. (c) MS/MS spectrum of the N-terminally trideutero-acetylated neo-N-terminus of topoII_α (1280AFKPIKKGKKR, Swiss-Prot accession: P11388) with the observed b- and y-type fragment ions indicated. (d) Schematic domain representation of human topoII_α. Proteomics identified GrM cleavage of topoII_α at Leu1280 in the regulatory C-terminal domain, within the first NLS. NES, nuclear export signal; TOPRIM, topoisomerase-primase domain; WHD, winged-helix domain
Figure 4
GrM efficiently and directly cleaves topoII_α_. (a) HeLa cells were incubated with 1 μ_M GrM or 1 μ_M GrM-SA, and 0.5 μ_g/ml SLO in the absence (dimethyl sulfoxide (DMSO)) or presence of 100 μ_M z-VAD-fmk for 6 h at 37 °C. TopoII_α cleavage was determined using immunoblot analysis. Hsp90_β was used as loading control. (b) 10 μ_g HeLa cell lysate was incubated at 37 °C with indicated concentrations of GrM or 50 nM GrM-SA for 30 min or with 5 nM GrM for the indicated lengths of time or 60 min with 5 nM GrM-SA. TopoII_α cleavage was determined using immunoblot analysis. β_-Tubulin was used as a loading control. (c) Twenty units of purified recombinant human topoII_α were incubated with 100 nM GrM or GrM-SA for 30 min at 37 °C. Cleavage was determined using immunoblot. (d) Cos7 cells were transiently transfected with enhanced green fluorescent protein (eGFP)-topoII_α and co-cultured with increasing effector:target (E:T) ratios of NK cells (KHYG1) for 4 h at 37 °C. Cleavage of eGFP-topoII_α was determined using immunoblot for eGFP. An arrow is used to indicate the topoII_α_ cleavage fragment
Figure 5
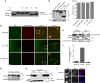
GrM disrupts topoII_α_ nuclear localization. (a) Twenty units of recombinant topoII_α_ were treated with 100 nM GrM or GrM-SA for 30 min at 37 °C (see also Figure 4c). These samples were incubated with 500 ng of supercoiled pUC18 plasmid for 30 min at 37 °C. pUC18 DNA relaxation was assessed on a 0.7% agarose gel. Supercoiled and relaxed pUC18 are indicated. (b) HeLa cells were transfected with full-length eGFP-topoII_α_ or topoII_α_ fragments that mimic GrM cleavage, that is, the N-terminal eGFP-topoII_α_ 1–1280 and C-terminal eGFP-topoII_α_ 1280–1531 fragments. Expression of the fragments was verified by immunoblotting for GFP. β_-Tubulin was used as a loading control. (c) Cells were transfected as in panel (b), and the cell viability was determined using PI staining. (d) Cells were transfected as in panel (b), and H2B-mCherry was co-transfected to stain nuclei. Localization was determined by fluorescent microscopy. (e) HeLa cells were incubated with 1 μ_M GrM and 0.5 μ_g/ml SLO for 30 min or mock treated, after which they were fractionated. Whole cell (WC) lysate, cytosolic (C), and nuclear (N) fractions were immunoblotted for topoII_α. An arrow is used to indicate the topoII_α cleavage fragment. (f) Semi-quantitative analysis was performed on the immunoblots of cells prepared in panel (e), and ratios of fragment/full-length topoII_α are plotted for WC, C, and N fractions. (g and h) HeLa cells were pretreated with 12 nM LMB for 2 h, after which they were treated with 0.5 μ_g/ml SLO in the absence or presence of 1 μ_M GrM. After a 30-min incubation, samples were immunoblotted for topoII_α (g). In addition, GrM-treated samples were fractionated and immunoblotted for topoII_α and for TAF5 and Hsp90_β_ (as controls for the nuclear and cytosolic fractions, respectively) (h). (i) HeLa cells were treated with 1 _μ_M GrM in the presence or absence of 0.5 _μ_g/ml SLO, followed by a 30-min incubation. Cells were then washed, fixed, stained, and GrM localization was visualized using confocal microscopy. DAPI (4,6-diamidino-2-phenylindole) was used to stain the nuclei
Figure 6
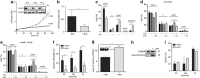
TopoII_α_ depletion phenotypically resembles GrM-induced apoptosis. (a) Treatment with 1 μ_g/ml dox for 48 h depleted topoII_α in HTETOP cells as determined using immunoblot (inset). Hsp90_β_ was used as loading control. HTETOP cells depleted of topoII_α_ for 48 h were seeded in the xCELLigence system, and CI was monitored in time (every 30 min for 96 h). Medium was refreshed after 48 h. Graph depicts n_=4 with mean±S.D. of a single experiment that is representative of three independent experiments. (b) The increase in CI (slope) between 12 and 96 h after seeding was determined for three independent xCELLigence experiments. (c) The cell cycle profile of HTETOP cells that had been depleted of topoII_α was determined 5 days after the seeding of the cells using PI-cell cycle flow cytometry. (d and e) Both wild-type (wt) and casp-8−/− Jurkat cells were treated in the presence of z-VAD-fmk with 1 _μ_M GrM or GrM-SA and 0.1 _μ_g/ml SLO for 24 h, after which cell cycle distributions were determined using PI-cell cycle flow cytometry. (f) Cell death of topoII_α_-depleted HTETOPs grown in the absence (dimethyl sulfoxide (DMSO)) or presence of 100 _μ_M z-VAD-fmk was determined at different time points after seeding using AnnV/PI flow cytometry. (g) Caspase-3/-7 activity was assessed in topoII_α_-depleted HTETOPs at 48 h after seeding using the CaspaseGlo assay. Nm23H1 served as a loading control. (h) Cells were seeded as in panel (g), and caspase-3 activation was detected using immunoblot. GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was used as a loading control. (i) Loss of mitochondrial membrane potential was measured at different time points after seeding of topoII_α_-depleted HTETOPs grown in the absence (DMSO) or presence of 100 _μ_M z-VAD-fmk. In all experiments, medium (supplemented with dox) was refreshed every 48 h
Figure 7
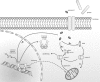
Schematic model of GrM-induced cell death. Perforin facilitates entry of GrM into the target cell via pore formation. Upon entry, GrM can cleave FADD, resulting in the recruitment and auto-processing of caspase-8, and subsequently activation of caspase-3 – either directly or via Bid-mediated targeting of the mitochondria and apoptosome formation. In addition, GrM can cleave nuclear topoII_α_, resulting in the formation of a C-terminal fragment that bears the NLS and remains in the nucleus, and an N-terminal fragment, which bears the catalytic domains, and translocates to the cytosol. The depletion of functional topoII_α_ in the nucleus results in G2/M cell cycle arrest, which also leads to mitochondrial perturbations and caspase-dependent cell death, via a yet to be identified mechanism. Furthermore, GrM can also cleave and inactivate survivin and hnRNP K, resulting in a decrease in the levels of caspase inhibitors, thereby facilitating caspase-induced apoptosis
Similar articles
- Intracellular serine protease inhibitor SERPINB4 inhibits granzyme M-induced cell death.
de Koning PJ, Kummer JA, de Poot SA, Quadir R, Broekhuizen R, McGettrick AF, Higgins WJ, Devreese B, Worrall DM, Bovenschen N. de Koning PJ, et al. PLoS One. 2011;6(8):e22645. doi: 10.1371/journal.pone.0022645. Epub 2011 Aug 3. PLoS One. 2011. PMID: 21857942 Free PMC article. - Geminin overexpression prevents the completion of topoisomerase IIα chromosome decatenation, leading to aneuploidy in human mammary epithelial cells.
Gardner L, Malik R, Shimizu Y, Mullins N, ElShamy WM. Gardner L, et al. Breast Cancer Res. 2011 May 19;13(3):R53. doi: 10.1186/bcr2884. Breast Cancer Res. 2011. PMID: 21595939 Free PMC article. - NK cell protease granzyme M targets alpha-tubulin and disorganizes the microtubule network.
Bovenschen N, de Koning PJ, Quadir R, Broekhuizen R, Damen JM, Froelich CJ, Slijper M, Kummer JA. Bovenschen N, et al. J Immunol. 2008 Jun 15;180(12):8184-91. doi: 10.4049/jimmunol.180.12.8184. J Immunol. 2008. PMID: 18523284 - Granzyme M: behind enemy lines.
de Poot SA, Bovenschen N. de Poot SA, et al. Cell Death Differ. 2014 Mar;21(3):359-68. doi: 10.1038/cdd.2013.189. Epub 2014 Jan 10. Cell Death Differ. 2014. PMID: 24413154 Free PMC article. Review. - Cytotoxic and non-cytotoxic roles of the CTL/NK protease granzyme B.
Afonina IS, Cullen SP, Martin SJ. Afonina IS, et al. Immunol Rev. 2010 May;235(1):105-16. doi: 10.1111/j.0105-2896.2010.00908.x. Immunol Rev. 2010. PMID: 20536558 Review.
Cited by
- Cytotoxic Lymphocytes Target HIV-1 Gag Through Granzyme M-Mediated Cleavage.
Saccon E, Mikaeloff F, Figueras Ivern P, Végvári Á, Sönnerborg A, Neogi U, van Domselaar R. Saccon E, et al. Front Immunol. 2021 Apr 19;12:669347. doi: 10.3389/fimmu.2021.669347. eCollection 2021. Front Immunol. 2021. PMID: 33953729 Free PMC article. - Killer cell proteases can target viral immediate-early proteins to control human cytomegalovirus infection in a noncytotoxic manner.
Shan L, Li S, Meeldijk J, Blijenberg B, Hendriks A, van Boxtel KJWM, van den Berg SPH, Groves IJ, Potts M, Svrlanska A, Stamminger T, Wills MR, Bovenschen N. Shan L, et al. PLoS Pathog. 2020 Apr 13;16(4):e1008426. doi: 10.1371/journal.ppat.1008426. eCollection 2020 Apr. PLoS Pathog. 2020. PMID: 32282833 Free PMC article. - Greek Fire, Poison Arrows, and Scorpion Bombs: How Tumor Cells Defend Against the Siege Weapons of Cytotoxic T Lymphocytes.
McKenzie B, Khazen R, Valitutti S. McKenzie B, et al. Front Immunol. 2022 May 3;13:894306. doi: 10.3389/fimmu.2022.894306. eCollection 2022. Front Immunol. 2022. PMID: 35592329 Free PMC article. Review. - Resistance of HIV-infected macrophages to CD8+ T lymphocyte-mediated killing drives activation of the immune system.
Clayton KL, Collins DR, Lengieza J, Ghebremichael M, Dotiwala F, Lieberman J, Walker BD. Clayton KL, et al. Nat Immunol. 2018 May;19(5):475-486. doi: 10.1038/s41590-018-0085-3. Epub 2018 Apr 18. Nat Immunol. 2018. PMID: 29670239 Free PMC article. - Perforin and granzymes: function, dysfunction and human pathology.
Voskoboinik I, Whisstock JC, Trapani JA. Voskoboinik I, et al. Nat Rev Immunol. 2015 Jun;15(6):388-400. doi: 10.1038/nri3839. Nat Rev Immunol. 2015. PMID: 25998963 Review.
References
- Lieberman J. Anatomy of a murder: how cytotoxic T cells and NK cells are activated, develop, and eliminate their targets. Immunol Rev. 2010;235:5–9. - PubMed
- Cullen SP, Brunet M, Martin SJ. Granzymes in cancer and immunity. Cell Death Differ. 2010;17:616–623. - PubMed
- Grossman WJ, Revell PA, Lu ZH, Johnson H, Bredemeyer AJ, Ley TJ. The orphan granzymes of humans and mice. Curr Opin Immunol. 2003;15:544–552. - PubMed
- Anthony DA, Andrews DM, Watt SV, Trapani JA, Smyth MJ. Functional dissection of the granzyme family: cell death and inflammation. Immunol Rev. 2010;235:73–92. - PubMed
- Bovenschen N, Kummer JA. Orphan granzymes find a home. Immunol Rev. 2010;235:117–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous