Suppression of iron-regulatory hepcidin by vitamin D - PubMed (original) (raw)
doi: 10.1681/ASN.2013040355. Epub 2013 Nov 7.
Joshua J Zaritsky, Jessica L Sea, Rene F Chun, Thomas S Lisse, Kathryn Zavala, Anjali Nayak, Katherine Wesseling-Perry, Mark Westerman, Bruce W Hollis, Isidro B Salusky, Martin Hewison
Affiliations
- PMID: 24204002
- PMCID: PMC3935584
- DOI: 10.1681/ASN.2013040355
Suppression of iron-regulatory hepcidin by vitamin D
Justine Bacchetta et al. J Am Soc Nephrol. 2014 Mar.
Abstract
The antibacterial protein hepcidin regulates the absorption, tissue distribution, and extracellular concentration of iron by suppressing ferroportin-mediated export of cellular iron. In CKD, elevated hepcidin and vitamin D deficiency are associated with anemia. Therefore, we explored a possible role for vitamin D in iron homeostasis. Treatment of cultured hepatocytes or monocytes with prohormone 25-hydroxyvitamin D or active 1,25-dihydroxyvitamin D decreased expression of hepcidin mRNA by 0.5-fold, contrasting the stimulatory effect of 25-hydroxyvitamin D or 1,25-dihydroxyvitamin D on related antibacterial proteins such as cathelicidin. Promoter-reporter and chromatin immunoprecipitation analyses indicated that direct transcriptional suppression of hepcidin gene (HAMP) expression mediated by 1,25-dihydroxyvitamin D binding to the vitamin D receptor caused the decrease in hepcidin mRNA levels. Suppression of HAMP expression was associated with a concomitant increase in expression of the cellular target for hepcidin, ferroportin protein, and decreased expression of the intracellular iron marker ferritin. In a pilot study with healthy volunteers, supplementation with a single oral dose of vitamin D (100,000 IU vitamin D2) increased serum levels of 25D-hydroxyvitamin D from 27±2 ng/ml before supplementation to 44±3 ng/ml after supplementation (P<0.001). This response was associated with a 34% decrease in circulating levels of hepcidin within 24 hours of vitamin D supplementation (P<0.05). These data show that vitamin D is a potent regulator of the hepcidin-ferroportin axis in humans and highlight a potential new strategy for the management of anemia in patients with low vitamin D and/or CKD.
Figures
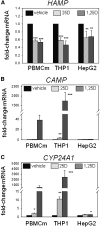
Figure 1.
Vitamin D suppresses expression of hepcidin (HAMP) in human monocytes and hepatocytes. Effect of in vitro treatment of PBMC monocytes (PBMCm), monocytic THP1 cells, and HepG2 hepatocytic cells with vehicle, 25D (100 nM), or 1,25D (5 nM) for 6 hours on expression of mRNA for HAMP (A), CAMP (B), and CYP24A1 (C). Data are shown as mean fold-change in gene expression (±SD) relative to vehicle (0.1% ethanol) controls. *P<0.05; **P<0.01; ***P<0.001, statistically different from vehicle-treated cells. For RT-PCR data, _n_=8 separate donors for PBMC monocytes, _n_=4 separate cultures of THP1 cells, and _n_=5 separate cultures of HepG2 cells.
Figure 2.
VDR-mediated suppression of hepcidin (HAMP) gene expression by 1,25D. (A) ChIP analysis of VDR and RNA Pol II interaction with the HAMP gene promoter. PBMC monocytes are treated with 1,25D (5 nM, 24 hours), chromatin extracts are prepared, and ChIP-grade antibodies are used to detect VDR and RNA Pol II interactions. The resulting enriched genomic DNA is qPCR amplified using primers for CYP24A1 (−1 kb from the transcription start site and −2 kb from the transcription start site), CAMP (−1 kb from the transcription start site), HAMP (−1 kb from the transcription start site). A negative control sequence for VDR and RNA Pol II (IGX1A) is also used. Data are shown first as mean arbitrary units for PCR amplification of DNA associated with RNA Pol II or VDR in cells treated with vehicle or 1,25D (mean of two separate chromatin preparations). In addition, fold-change values are presented showing the change in RNA Pol II or VDR binding to DNA after treatment with 1,25D relative to vehicle-treated cells. (B) Effect of 25D (100 nM) and 1,25D (5 nM) on HAMP promoter-reporter activity in VDR-expressing MC3T3 mouse osteoblastic cells. Data are shown as HAMP target gene firefly/Renilla housekeeping luciferase activity (×10−3). *P<0.05, statistically different from vehicle-treated cells.
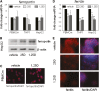
Figure 3.
Effect of vitamin D metabolites on ferroportin and ferritin expression in human monocytes and hepatocytes. (A) Effect of in vitro treatment of PBMC monocytes (PBMCm), monocytic THP1 cells, and HepG2 hepatocytic cells with vehicle, 25D (100 nM) or 1,25D (5 nM) for 6 hours on ferroportin mRNA expression. Data are shown as mean fold-change in gene expression (±SD relative to vehicle [0.1% ethanol] controls). (B) Western blot analysis of protein for ferroportin protein in HepG2 cells treated with vehicle, 25D (100 nM), or 1,25D (5 nM) for 24 hours. Loading is normalized by analysis of the housekeeping protein _β_-actin. (C) Immunohistochemical analysis of ferroportin protein in PBMC monocytes after treatment with vehicle or 1,25D (5 nM) for 24 hours. Ferroportin protein is shown in red with 4′,6-diamidino-2-phenylindole (DAPI) staining of nuclei shown in blue. (D) Effect of in vitro treatment of PBMC monocytes, THP1 cells, and HepG2 cells with vehicle, 25D (100 nM), or 1,25D (5 nM) for 6 hours on ferritin mRNA expression. Data are shown as mean fold-change in gene expression (±SD relative to vehicle controls). (E) Immunohistochemical analysis of ferritin protein in HepG2 cells after treatment with vehicle, 25D (100 nM), or 1,25D (5 nM) for 24 hours. Ferritin protein is shown in red with DAPI staining of nuclei in blue. *P<0.05; **P<0.01; ***P<0.001, statistically different from vehicle-treated cells. For RT-PCR data, _n_=8 separate donors for PBMC monocytes, _n_=4 separate cultures of THP1 cells, and _n_=5 separate cultures of HepG2 cells.
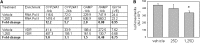
Figure 4.
Effects of supplementation with vitamin D2 on circulating hepcidin levels in healthy humans. A single-arm pharmacokinetic study is performed in seven healthy volunteers (four men; median age 42 years; range, 27–63) to assess changes in serum levels of hepcidin after a single dose of oral vitamin D2 (100,000 IU). Two blood samples are drawn before supplementation and two are drawn after supplementation. Serum concentrations of 25D (ng/ml) (A), 1,25D (pg/ml) (B), and hepcidin (ng/ml) (C). Data are shown as the mean±SEM. Experimental means were compared statistically using a paired t test.*P<0.05; **P<0.01; ***P<0.001, statistically different from baseline values.
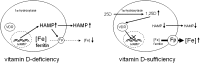
Figure 5.
Vitamin D and the hepcidin-ferroportin iron-regulatory axis. Schematic representation of a proposed mechanism for vitamin D regulation of hepcidin/HAMP – ferroportin (Fp) interaction in hepatocytes and monocytes. Under conditions of vitamin D deficiency, elevated synthesis of hepcidin by hepatocytes or monocytes may increase intracellular and systemic concentrations of hepcidin and decrease membrane expression of Fp in these cells. The resulting suppression of iron export will, in turn, lead to intracellular accumulation, increased cellular ferritin, and decreased systemic levels of iron. Under conditions of vitamin D sufficiency, decreased transcription of HAMP may lead to decreased intracellular and systemic concentrations of hepcidin and concomitant increased membrane expression of Fp. The resulting enhancement of iron export may then lead to decreased intracellular iron and ferritin and increased systemic levels of iron.
Similar articles
- Antibacterial responses by peritoneal macrophages are enhanced following vitamin D supplementation.
Bacchetta J, Chun RF, Gales B, Zaritsky JJ, Leroy S, Wesseling-Perry K, Boregaard N, Rastogi A, Salusky IB, Hewison M. Bacchetta J, et al. PLoS One. 2014 Dec 30;9(12):e116530. doi: 10.1371/journal.pone.0116530. eCollection 2014. PLoS One. 2014. PMID: 25549329 Free PMC article. - Targeting the hepcidin-ferroportin pathway in anaemia of chronic kidney disease.
Sheetz M, Barrington P, Callies S, Berg PH, McColm J, Marbury T, Decker B, Dyas GL, Truhlar SME, Benschop R, Leung D, Berg J, Witcher DR. Sheetz M, et al. Br J Clin Pharmacol. 2019 May;85(5):935-948. doi: 10.1111/bcp.13877. Epub 2019 Mar 4. Br J Clin Pharmacol. 2019. PMID: 30677788 Free PMC article. Clinical Trial. - Bone marrow iron distribution, hepcidin, and ferroportin expression in renal anemia.
Bârsan L, Stanciu A, Stancu S, Căpuşă C, Brătescu L, Mandache E, Radu E, Mircescu G. Bârsan L, et al. Hematology. 2015 Oct;20(9):543-52. doi: 10.1179/1607845415Y.0000000004. Epub 2015 Mar 8. Hematology. 2015. PMID: 25745821 - The molecular basis of iron overload disorders and iron-linked anemias.
Kaplan J, Ward DM, De Domenico I. Kaplan J, et al. Int J Hematol. 2011 Jan;93(1):14-20. doi: 10.1007/s12185-010-0760-0. Epub 2011 Jan 7. Int J Hematol. 2011. PMID: 21210258 Review. - Hepcidin and iron homeostasis.
Ganz T, Nemeth E. Ganz T, et al. Biochim Biophys Acta. 2012 Sep;1823(9):1434-43. doi: 10.1016/j.bbamcr.2012.01.014. Epub 2012 Jan 26. Biochim Biophys Acta. 2012. PMID: 22306005 Free PMC article. Review.
Cited by
- Elevated plasma hepcidin concentrations are associated with an increased risk of mortality and nonfatal cardiovascular events in patients with type 2 diabetes: a prospective study.
Mantovani A, Busti F, Borella N, Scoccia E, Pecoraro B, Sani E, Morandin R, Csermely A, Piasentin D, Grespan E, Castagna A, Bilson J, Byrne CD, Valenti L, Girelli D, Targher G. Mantovani A, et al. Cardiovasc Diabetol. 2024 Aug 17;23(1):305. doi: 10.1186/s12933-024-02377-x. Cardiovasc Diabetol. 2024. PMID: 39154180 Free PMC article. - Effect of Single High-Dose Vitamin D3 Supplementation on Post-Ultra Mountain Running Heart Damage and Iron Metabolism Changes: A Double-Blind Randomized Controlled Trial.
Stankiewicz B, Mieszkowski J, Kochanowicz A, Brzezińska P, Niespodziński B, Kowalik T, Waldziński T, Kowalski K, Borkowska A, Reczkowicz J, Daniłowicz-Szymanowicz L, Antosiewicz J. Stankiewicz B, et al. Nutrients. 2024 Jul 31;16(15):2479. doi: 10.3390/nu16152479. Nutrients. 2024. PMID: 39125358 Free PMC article. Clinical Trial. - Association between anemia and vitamin D deficiency in German seniors : A retrospective data analysis.
Schuchart DM, Becker I, Harbeck B, Röhrig G. Schuchart DM, et al. Z Gerontol Geriatr. 2024 Nov;57(7):563-568. doi: 10.1007/s00391-024-02322-3. Epub 2024 Jul 5. Z Gerontol Geriatr. 2024. PMID: 38967671 English. - Continuous training in young athletes decreases hepcidin secretion and is positively correlated with serum 25(OH)D and ferritin.
Kobayashi Y, Taniguchi R, Shirasaki E, Yoshimoto YS, Aoi W, Kuwahata M. Kobayashi Y, et al. PeerJ. 2024 Jun 27;12:e17566. doi: 10.7717/peerj.17566. eCollection 2024. PeerJ. 2024. PMID: 38948227 Free PMC article. - Alterations in regulators of the renal-bone axis, inflammation and iron status in older people with early renal impairment and the effect of vitamin D supplementation.
Christodoulou M, Aspray TJ, Piec I, Fraser WD, Schoenmakers I; VDOP Trial group. Christodoulou M, et al. Age Ageing. 2024 May 1;53(5):afae096. doi: 10.1093/ageing/afae096. Age Ageing. 2024. PMID: 38770543 Free PMC article.
References
- Kalantar-Zadeh K, Streja E, Miller JE, Nissenson AR: Intravenous iron versus erythropoiesis-stimulating agents: Friends or foes in treating chronic kidney disease anemia? Adv Chronic Kidney Dis 16: 143–151, 2009 - PubMed
- Nakanishi T, Hasuike Y, Otaki Y, Kida A, Nonoguchi H, Kuragano T: Hepcidin: another culprit for complications in patients with chronic kidney disease? Nephrol Dial Transplant 26: 3092–3100, 2011 - PubMed
- Young B, Zaritsky J: Hepcidin for clinicians. Clin J Am Soc Nephrol 4: 1384–1387, 2009 - PubMed
- Ganz T: Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102: 783–788, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK 67563/DK/NIDDK NIH HHS/United States
- UL1TR000124/TR/NCATS NIH HHS/United States
- R21 DK091672/DK/NIDDK NIH HHS/United States
- R01 DK035423/DK/NIDDK NIH HHS/United States
- KL2 TR000122/TR/NCATS NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- DK 35423/DK/NIDDK NIH HHS/United States
- T32 GM007185/GM/NIGMS NIH HHS/United States
- KL2TR000122/TR/NCATS NIH HHS/United States
- UL1 RR033176/RR/NCRR NIH HHS/United States
- DK0911672/DK/NIDDK NIH HHS/United States
- UL1 RR-033176/RR/NCRR NIH HHS/United States
- R01 DK067563/DK/NIDDK NIH HHS/United States
- P30 AI028697/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical