Cinnamon treatment upregulates neuroprotective proteins Parkin and DJ-1 and protects dopaminergic neurons in a mouse model of Parkinson's disease - PubMed (original) (raw)
Cinnamon treatment upregulates neuroprotective proteins Parkin and DJ-1 and protects dopaminergic neurons in a mouse model of Parkinson's disease
Saurabh Khasnavis et al. J Neuroimmune Pharmacol. 2014 Sep.
Abstract
Upregulation and/or maintenance of Parkinson's disease (PD)-related beneficial proteins such as Parkin and DJ-1 in astrocytes during neurodegenerative insults may have therapeutic efficacy in PD. Cinnamon is a commonly used natural spice and flavoring material throughout the world. Here we have explored a novel use of cinnamon in upregulating Parkin and DJ-1 and protecting dopaminergic neurons in MPTP mouse model of PD. Recently we have delineated that oral feeding of cinnamon (Cinnamonum verum) powder produces sodium benzoate (NaB) in blood and brain of mice. Proinflammatory cytokine IL-1β decreased the level of Parkin/DJ-1 in mouse astrocytes. However, cinnamon metabolite NaB abrogated IL-1β-induced loss of these proteins. Inability of TNF-α to produce nitric oxide (NO) and decrease the level of Parkin/DJ-1 in wild type (WT) astrocytes, failure of IL-1β to reduce Parkin/DJ-1 in astrocytes isolated from iNOS (-/-) mice, and decrease in Parkin/DJ-1 in WT astrocytes by NO donor DETA-NONOate suggest that NO is a negative regulator of Parkin/DJ-1. Furthermore, suppression of IL-1β-induced expression of iNOS in astrocytes by NaB and reversal of NaB-mediated protection of Parkin/DJ-1 by DETA-NONOate in astrocytes indicate that NaB protects Parkin/DJ-1 in activated astrocytes via suppressing iNOS. Similarly MPTP intoxication also increased the level of iNOS and decreased the level of Parkin/DJ-1 in vivo in the nigra. However, oral treatment of MPTP-intoxicated mice with cinnamon powder and NaB reduced the expression of iNOS and protected Parkin/DJ-1 in the nigra. These findings paralleled dopaminergic neuronal protection, normalized striatal neurotransmitters, and improved motor functions by cinnamon in MPTP-intoxicated mice. These results suggest that cinnamon may be beneficial for PD patients.
Figures
Fig. 1
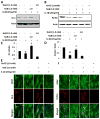
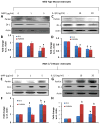
Effect of sodium benjoate (NaB) on IL-1β-mediated down-regulation of DJ-1 and Parkin in mouse primary astrocytes. Mouse astrocytes pre-treated with NaB for 6 h were stimulated with IL-1β for 18 h under serum-free condition. Sodium formate (NaFO) was used as a negative control for NaB. Western blot was performed to analyze the levels of DJ-1 (a) and Parkin (b). Actin was used as a house keeping protein. Densitometric analysis was performed and results presented as protein expression relative to actin (DJ-1, c; Parkin, d). Results are mean±SD of three different experiments. a p<0.05 vs control; b p<0.001 versus control; c p<0.001 vs IL-1β. After similar treatments, astrocytes were _double_-labeled for GFAP & DJ-1 (e) and GFAP & Parkin (f)
Fig. 2
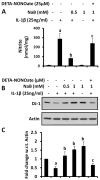
Effect of NaB on IL-1β-mediated production of nitric oxide in mouse primary astrocytes. Mouse astrocytes pre-treated with NaB for 6 h were stimulated with IL-1β for 18 h under serum-free condition in the presence or absence of DETA-NONOate (25 μM). Levels of nitrite (a) were measured in supernatants by Griess reagent. Expression of DJ-1 protein (b) was monitored in cells by Western blot. Densitometric analysis was performed and results presented as protein expression relative to actin (c). Results are mean±SD of three different experiments. a p<0.001 vs control; b p<0.001 vs IL-1β; c p<0.05 vs NaB
Fig. 3
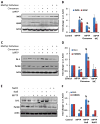
Involvement of nitric oxide in IL-1β-mediated down-regulation of DJ-1 and Parkin in mouse primary astrocytes. Mouse astrocytes were stimulated with IL-1β in the presence of different concentrations of L-NIL and PTIO for 24 h followed by monitoring the expression of DJ-1 and Parkin by Western blot (a). Densitometric analysis was performed and results presented as relative to actin (b). Cells were also treated with different concentrations of DETA-NONOate for 24 h followed by monitoring levels of DJ-1 and Parkin by Western blot analysis (c). Densitometric analysis was performed and results presented as relative to actin (d). Results are mean±SD of three different experiments. a p<0.001 versus control; b p<0.001 vs IL-1β
Fig. 4
Effect of TNF-α on the expression of DJ-1 and Parkin in mouse primary astrocytes. Cells were stimulated with TNF-α for different time periods under serum-free condition followed by monitoring levels of nitrite in supernatants (a). Levels of DJ-1 and Parkin were monitored in cells by Western blot analysis (b). Densitometric analysis was performed and results presented as relative to actin (c). Results are mean±SD of three different experiments. a p<0.001 versus control. After 18 h of stimulation with TNF-α, cells were _double_-labeled for GFAP and DJ-1 (d). Results represent three independent analyses
Fig. 5
The role of inducible nitric oxide synthase (iNOS) in the regulation of DJ-1 and Parkin in mouse primary astrocytes. (a–d) Astrocytes isolated from wild-type mice were stimulated with MPP+ and IL-1β for 24 h followed by monitoring levels of DJ-1 and Parkin by Western blot analysis (a, MPP+; c, IL-1β). Densitometric analysis was performed and results presented as relative to actin (b, MPP+; d, IL-1β). (e–h) Astrocytes isolated from iNOS (−/−) mice were stimulated with MPP+ and IL-1β for 24 h followed by monitoring levels of DJ-1 and Parkin by Western blot analysis (E, MPP+; G, IL-1β). Densitometric analysis was performed and results presented as relative to actin (f, MPP+; h, IL-1β). Results are mean±SD of three different experiments. a p<0.001 vs WT-control; b p<0.001 vs iNOS (−/−)-control
Fig. 6
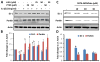
Effect of cinnamon and its metabolite NaB on the expression of DJ-1 and Parkin in vivo in the nigra of MPTP-intoxicated mice. Mice (_n_=4) receiving cinnamon powder (100 mg/kg body wt/d) or vehicle (methyl cellulose) from 3 h after the last injection of MPTP were sacrificed 7 days after the last injection of MPTP followed by monitoring levels of iNOS & GFAP (a) and DJ-1 & Parkin (c) by Western blot. Densitometric analysis was performed and results presented as relative to actin (b, iNOS & GFAP; d, DJ-1 & Parkin). Mice (_n_=4) receiving NaB (50 mg/kg body wt/d) or NaFO (negative control) from 3 h after the last injection of MPTP were sacrificed 7 days after the last injection of MPTP followed by monitoring levels of DJ-1 & Parkin (E) by Western blot. Densitometric analysis was performed and results presented as relative to actin (f). Results are mean±SEM of four mice per group. a p<0.001 vs control; b p<0.001 vs MPTP
Fig. 7
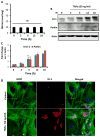
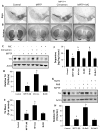
Cinnamon and its metabolite NaB protect dopaminergic neurons in MPTP-intoxicated mice. Mice receiving cinnamon powder (100 mg/kg body wt/d) or vehicle from 3 h after the last injection of MPTP were sacrificed 7 days after the last injection of MPTP followed by TH immunostaining of SNpc (a) and striatum (b), quantification of nigral TH (c, Western blot; d, densitometric analysis), quantification of TH-positive fibers in striatum (e), and assay of neurotransmitters in striatum (f). Similarly, mice receiving NaB (50 mg/kg body wt/d) or NaFO from 3 h after the last injection of MPTP were sacrificed 7 days after the last injection of MPTP followed by quantification of nigral TH (g, Western blot; h, densitometric analysis) and assay of neurotransmitters in striatum (f). Data are means±SEM of six mice per group. ap<0.001 vs control; bp<0.001 vs MPTP
Fig. 8
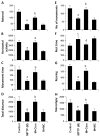
Cinnamon treatment improves motor functions in MPTP-intoxicated mice. Mice receiving cinnamon powder (100 mg/kg body wt/d) or vehicle from 3 h after the last injection of MPTP were tested for motor functions (a, rotorod; b, horizontal activity; c, movement time; d, total distance; e, number of movements; f, rest time; g, rearing; h, stereotypy) 7 days after the last injection of MPTP. Data are means±SEM of six mice per group. a_p_<0.001 vs control; c_p_<0.05 vs control; b_p_<0.001 vs MPTP; d_p_<0.05 vs MPTP
Comment in
Similar articles
- Cinnamon and its Metabolite Protect the Nigrostriatum in a Mouse Model of Parkinson's Disease Via Astrocytic GDNF.
Patel D, Jana A, Roy A, Pahan K. Patel D, et al. J Neuroimmune Pharmacol. 2019 Sep;14(3):503-518. doi: 10.1007/s11481-019-09855-0. Epub 2019 May 22. J Neuroimmune Pharmacol. 2019. PMID: 31119595 Free PMC article. - Tinospora cordifolia Suppresses Neuroinflammation in Parkinsonian Mouse Model.
Birla H, Rai SN, Singh SS, Zahra W, Rawat A, Tiwari N, Singh RK, Pathak A, Singh SP. Birla H, et al. Neuromolecular Med. 2019 Mar;21(1):42-53. doi: 10.1007/s12017-018-08521-7. Epub 2019 Jan 14. Neuromolecular Med. 2019. PMID: 30644041 - Neuroprotective potential of cinnamon and its metabolites in Parkinson's disease: Mechanistic insights, limitations, and novel therapeutic opportunities.
Angelopoulou E, Paudel YN, Piperi C, Mishra A. Angelopoulou E, et al. J Biochem Mol Toxicol. 2021 Apr;35(4):e22720. doi: 10.1002/jbt.22720. Epub 2021 Jan 24. J Biochem Mol Toxicol. 2021. PMID: 33491302 Review. - Mechanisms of MPTP toxicity and their implications for therapy of Parkinson's disease.
Watanabe Y, Himeda T, Araki T. Watanabe Y, et al. Med Sci Monit. 2005 Jan;11(1):RA17-23. Med Sci Monit. 2005. PMID: 15614202 Review.
Cited by
- Ocrelizumab alters the circulating metabolome in people with relapsing-remitting multiple sclerosis.
Siavoshi F, Ladakis DC, Muller A, Nourbakhsh B, Bhargava P. Siavoshi F, et al. Ann Clin Transl Neurol. 2024 Sep;11(9):2485-2498. doi: 10.1002/acn3.52167. Epub 2024 Aug 26. Ann Clin Transl Neurol. 2024. PMID: 39185939 Free PMC article. - Neurodegenerative Etiology of Aromatic L-Amino Acid Decarboxylase Deficiency: a Novel Concept for Expanding Treatment Strategies.
Sternberg Z. Sternberg Z. Mol Neurobiol. 2024 May;61(5):2996-3018. doi: 10.1007/s12035-023-03684-2. Epub 2023 Nov 13. Mol Neurobiol. 2024. PMID: 37953352 Review. - The Involvement of Neuroinflammation in the Onset and Progression of Parkinson's Disease.
Jurcau A, Andronie-Cioara FL, Nistor-Cseppento DC, Pascalau N, Rus M, Vasca E, Jurcau MC. Jurcau A, et al. Int J Mol Sci. 2023 Sep 26;24(19):14582. doi: 10.3390/ijms241914582. Int J Mol Sci. 2023. PMID: 37834030 Free PMC article. Review. - Role of Astrogliosis in the Pathogenesis of Parkinson's Disease: Insights into Astrocytic Nrf2 Pathway as a Potential Therapeutic Target.
Bhushan B, Singh NK. Bhushan B, et al. CNS Neurol Disord Drug Targets. 2024;23(8):1015-1029. doi: 10.2174/0118715273270473231002104610. CNS Neurol Disord Drug Targets. 2024. PMID: 37817521 Review. - Analysis of bioactive compounds in cinnamon leaves and preparation of nanoemulsion and byproducts for improving Parkinson's disease in rats.
Wang YC, Wang V, Chen BH. Wang YC, et al. Front Nutr. 2023 Aug 2;10:1229192. doi: 10.3389/fnut.2023.1229192. eCollection 2023. Front Nutr. 2023. PMID: 37599679 Free PMC article.
References
- Abd El-Mawla AM, Schmidt W, Beerhues L. Cinnamic acid is a precursor of benzoic acids in cell cultures of Hypericum androsaemum L. but not in cell cultures of Centaurium erythraea RAFN. Planta. 2001;212:288–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical