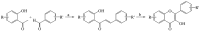Flavonoids with M1 muscarinic acetylcholine receptor binding activity - PubMed (original) (raw)
Flavonoids with M1 muscarinic acetylcholine receptor binding activity
Meyyammai Swaminathan et al. Molecules. 2014.
Abstract
Muscarinic acetylcholine receptor-active compounds have potential for the treatment of Alzheimer's disease. In this study, a series of natural and synthetic flavones and flavonols was assayed in vitro for their ability to inhibit radioligand binding at human cloned M1 muscarinic receptors. Several compounds were found to possess competitive binding affinity (Ki=40-110 µM), comparable to that of acetylcholine (Ki=59 µM). Despite the fact that these compounds lack a positively-charged ammonium group under physiological conditions, molecular modelling studies suggested that they bind to the orthosteric site of the receptor, mainly through non-polar interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
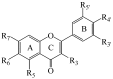
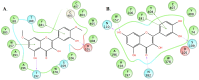
Figure 1
General structure of the investigated flavones and flavonols.
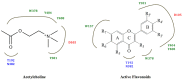
Scheme 1
Synthesis of flavones 1–4.
Scheme 2
Synthesis of flavonols 9–10.
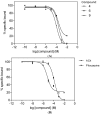
Figure 2
Displacement curves for the binding of [3H]N-methylscopolamine to the M1 mAChR in the presence of (A) 3',4',5',5,6,7-hexamethoxyflavone (4), luteolin (6) and ombuin (9) and (B) acetylcholine (ACh) and pilocarpine. Receptor membranes (13 µg protein/well) were incubated with N-methylscopolamine (0.2 nM) in the presence of the compounds at 27 °C for 120 min. Each data point is expressed as a mean ± SEM (n = 2).
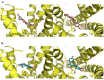
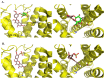
Figure 3
3D representations of the docking poses of (A) ombuin (9) (brown) and (B) quercetin (12) (blue-green) in complex with the human M1 mAChR model obtained from induced-fit docking using Glide v5.7 and Prime v3.0 (Schrödinger LLC) with side views and top views (from the extracellular surface) depicted in the left hand and right hand panels, respectively. For the purpose of clarity, only D105 and W378 from among the active site residues are depicted in stick representation and some of the transmembrane helices are not shown.
Figure 4
2D representations of the ligand-receptor interactions of (A) ombuin (9) and (B) quercetin (12) in complex with the human M1 mAChR model obtained from induced-fit docking using Glide v5.7 and Prime v3.0 (Schrödinger LLC), showing pi-pi stacking interactions (green lines), hydrogen bonds involving backbone atoms (solid purple arrows) and hydrogen bonds involving side-chain atoms (dashed purple arrows). Negatively-charged, polar and hydrophobic residues are depicted with red, light blue and green circles, respectively.
Figure 5
The structural binding analysis map for acetylcholine and a composite map for the active flavonoids, 3',4',5',5,6,7-hexamethoxyflavone (4), luteolin (6) and ombuin (9), obtained from molecular modelling studies. Negatively-charged, polar and hydrophobic residues are depicted in red, blue and dark green, respectively.
Figure 6
3D representations of the docking poses of (A) ombuin-3'-glucuronide and (B) ombuin-3'-sulfate in complex with the human M1 mAChR model obtained from induced-fit docking using Glide v5.7 and Prime v3.0 (Schrödinger LLC) with side views and top views (from the extracellular surface) depicted in the left hand and right hand panels, respectively. For the purpose of clarity, only D105 and W378 from among the active site residues are depicted in stick representation and some of the transmembrane helices are not shown. The carbon atoms of the glucuronide moiety in ombuin-3'-glucuronide are coloured in light green.
Similar articles
- The predicted 3D structures of the human M1 muscarinic acetylcholine receptor with agonist or antagonist bound.
Peng JY, Vaidehi N, Hall SE, Goddard WA 3rd. Peng JY, et al. ChemMedChem. 2006 Aug;1(8):878-90. doi: 10.1002/cmdc.200600047. ChemMedChem. 2006. PMID: 16902941 - Progress toward a high-affinity allosteric enhancer at muscarinic M1 receptors.
Lazareno S, Popham A, Birdsall NJ. Lazareno S, et al. J Mol Neurosci. 2003;20(3):363-7. doi: 10.1385/JMN:20:3:363. J Mol Neurosci. 2003. PMID: 14501021 - Enantiomeric N-methyl-4-piperidyl benzilates as muscarinic receptor ligands: Radioligand binding studies and docking studies to models of the three muscarinic receptors M1, M2 and M3.
Selent J, Brandt W, Pamperin D, Göber B. Selent J, et al. Bioorg Med Chem. 2006 Mar 15;14(6):1729-36. doi: 10.1016/j.bmc.2005.10.030. Epub 2005 Nov 14. Bioorg Med Chem. 2006. PMID: 16290166 - SAR studies on carboxylic acid series M(1) selective positive allosteric modulators (PAMs).
Kuduk SD, Beshore DC. Kuduk SD, et al. Curr Top Med Chem. 2014;14(15):1738-54. doi: 10.2174/1568026614666140826120224. Curr Top Med Chem. 2014. PMID: 25176125 Review. - Importance and prospects for design of selective muscarinic agonists.
Jakubík J, Michal P, Machová E, Dolezal V. Jakubík J, et al. Physiol Res. 2008;57 Suppl 3:S39-S47. doi: 10.33549/physiolres.931449. Epub 2008 May 13. Physiol Res. 2008. PMID: 18481916 Review.
Cited by
- Quercetin promotes gastrointestinal motility and mucin secretion in loperamide-induced constipation of SD rats through regulation of the mAChRs downstream signal.
Kim JE, Lee MR, Park JJ, Choi JY, Song BR, Son HJ, Choi YW, Kim KM, Hong JT, Hwang DY. Kim JE, et al. Pharm Biol. 2018 Dec;56(1):309-317. doi: 10.1080/13880209.2018.1474932. Pharm Biol. 2018. PMID: 29952685 Free PMC article. - In Silico and In Vitro Analysis of Bacoside A Aglycones and Its Derivatives as the Constituents Responsible for the Cognitive Effects of Bacopa monnieri.
Ramasamy S, Chin SP, Sukumaran SD, Buckle MJ, Kiew LV, Chung LY. Ramasamy S, et al. PLoS One. 2015 May 12;10(5):e0126565. doi: 10.1371/journal.pone.0126565. eCollection 2015. PLoS One. 2015. PMID: 25965066 Free PMC article. - Flavonoids: an overview.
Panche AN, Diwan AD, Chandra SR. Panche AN, et al. J Nutr Sci. 2016 Dec 29;5:e47. doi: 10.1017/jns.2016.41. eCollection 2016. J Nutr Sci. 2016. PMID: 28620474 Free PMC article. Review. - Flavonoids: structure-function and mechanisms of action and opportunities for drug development.
Safe S, Jayaraman A, Chapkin RS, Howard M, Mohankumar K, Shrestha R. Safe S, et al. Toxicol Res. 2021 Jan 20;37(2):147-162. doi: 10.1007/s43188-020-00080-z. eCollection 2021 Apr. Toxicol Res. 2021. PMID: 33868973 Free PMC article. Review. - Potential Therapeutic Targets of Quercetin and Its Derivatives: Its Role in the Therapy of Cognitive Impairment.
Jakaria M, Azam S, Jo SH, Kim IS, Dash R, Choi DK. Jakaria M, et al. J Clin Med. 2019 Oct 25;8(11):1789. doi: 10.3390/jcm8111789. J Clin Med. 2019. PMID: 31717708 Free PMC article. Review.
References
- Langmead C.J., Watson J., Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 2008;117:232–243. - PubMed
- Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. - PubMed
- Chun O.K., Chung S.J., Song W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007;137:1244–1252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources