Multidrug resistance protein 1 (MRP1, ABCC1), a "multitasking" ATP-binding cassette (ABC) transporter - PubMed (original) (raw)
Review
Multidrug resistance protein 1 (MRP1, ABCC1), a "multitasking" ATP-binding cassette (ABC) transporter
Susan P C Cole. J Biol Chem. 2014.
Abstract
The multidrug resistance protein 1 (MRP1) encoded by ABCC1 was originally discovered as a cause of multidrug resistance in tumor cells. However, it is now clear that MRP1 serves a broader role than simply mediating the ATP-dependent efflux of drugs from cells. The antioxidant GSH and the pro-inflammatory cysteinyl leukotriene C4 have been identified as key physiological organic anions effluxed by MRP1, and an ever growing body of evidence indicates that additional lipid-derived mediators are also substrates of this transporter. As such, MRP1 is a multitasking transporter that likely influences the etiology and progression of a host of human diseases.
Keywords: ABC Transporter; Inflammation; Leukotriene; Lipid Signaling; Lipid Transport; Lysophospholipid; MRP1; Multidrug Transporter; Organic Anion Transport; Oxidative Stress.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
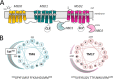
FIGURE 1.
Topology of MRP1 and projections of amphipathic TM α-helices 6 and 17. A, shown is a schematic diagram of a predicted secondary structure of MRP1/ABCC1. The positions of TMs 6, 10, 11, 16, and 17, which have been identified as containing key determinants of MRP1 substrate specificity, are highlighted. Also indicated are CL5 and CL7, which contain amino acids involved in substrate specificity, proper folding, and plasma membrane trafficking, as well as the transport mechanism of MRP1. B, shown are helical wheel projections of 18 amino acids of the amphipathic TM6 (in MSD1) and TM17 (in MSD2) of MRP1. The clustering on one side of each TM helix of polar residues with side chains capable of H-bonding is indicated by the shaded curve. Highlighted on the projection of TM6 is Lys332, which is particularly critical for LTC4 and GSH transport (43, 51).
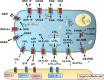
FIGURE 2.
ATP-dependent MRP1-mediated transport of endogenous and xenobiotic solutes. The diagram illustrates the diversity of molecules effluxed by MRP1 across the plasma membrane in an ATP-dependent fashion. Included are representative examples of the endogenous/physiological substrates, both proposed and demonstrated, effluxed by MRP1 such as lipid-derived effectors and mediators of cell signaling (and their receptors) implicated in the etiology and progression of multiple human diseases. The best characterized physiologic substrate of MRP1, viz. LTC4, is highlighted, and only in this case is the dependence of transport on ATP depicted. Also shown are the multiple and complex roles of GSH, both as a transported solute and as a stimulant of the transport of other solutes by MRP1. The ATP dependence of MRP1-mediated transport of these molecules is not depicted due to space constraints but is understood. EP1–4 refers to the four subtypes of PGE2 receptors. Abbreviations: GS-DHN, GS-1,4-dihydroxy-nonene; GS-HNEA, GS-4-hydroxy-2-nonenoic acid; BF, bioflavonoid; LTCS, LTC4 synthase; LTD4, leukotriene D4; LTE4, leukotriene E4; COA, conjugated organic anion; dPGJ2, 15-deoxy-Δ(12,14) PGJ2; S1PR1–5, S1P receptors 1–5; UCOA, unconjugated organic anion; VRP, verapamil; 'X', hydrophobic xenobiotic or drug.
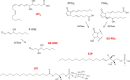
FIGURE 3.
Signaling molecules derived from endogenous lipids and transported by MRP1. Shown are the chemical structures of lipid-derived signaling molecules and their GSH conjugates that are reported to be transported by MRP1. Abbreviations: GS-HNE, GSH-conjugated 4-HNE; GS-PGs, GSH-conjugated prostaglandins; dPGJ2, 15-deoxy-Δ(12,14) prostaglandin J2; PGA2, prostaglandin A2.
Similar articles
- Targeting multidrug resistance protein 1 (MRP1, ABCC1): past, present, and future.
Cole SP. Cole SP. Annu Rev Pharmacol Toxicol. 2014;54:95-117. doi: 10.1146/annurev-pharmtox-011613-135959. Epub 2013 Sep 18. Annu Rev Pharmacol Toxicol. 2014. PMID: 24050699 Review. - Analysis of human multidrug resistance protein 1 (ABCC1) by matrix-assisted laser desorption ionization/time of flight mass spectrometry: toward identification of leukotriene C4 binding sites.
Wu P, Oleschuk CJ, Mao Q, Keller BO, Deeley RG, Cole SP. Wu P, et al. Mol Pharmacol. 2005 Nov;68(5):1455-65. doi: 10.1124/mol.105.016576. Epub 2005 Aug 16. Mol Pharmacol. 2005. PMID: 16105987 - Functional reconstitution of substrate transport by purified multidrug resistance protein MRP1 (ABCC1) in phospholipid vesicles.
Mao Q, Deeley RG, Cole SP. Mao Q, et al. J Biol Chem. 2000 Nov 3;275(44):34166-72. doi: 10.1074/jbc.M004584200. J Biol Chem. 2000. PMID: 10942765 - Transport of glutathione and glutathione conjugates by MRP1.
Cole SP, Deeley RG. Cole SP, et al. Trends Pharmacol Sci. 2006 Aug;27(8):438-46. doi: 10.1016/j.tips.2006.06.008. Epub 2006 Jul 3. Trends Pharmacol Sci. 2006. PMID: 16820223 Review.
Cited by
- Cell-to-cell communications of cGAS-STING pathway in tumor immune microenvironment.
Wang M, Xu P, Wu Q. Wang M, et al. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Jan 12;53(1):15-24. doi: 10.3724/zdxbyxb-2023-0482. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38229499 Free PMC article. Review. Chinese, English. - Heparin and Heparin-Based Drug Delivery Systems: Pleiotropic Molecular Effects at Multiple Drug Resistance of Osteosarcoma and Immune Cells.
Todosenko N, Yurova K, Khaziakhmatova O, Malashchenko V, Khlusov I, Litvinova L. Todosenko N, et al. Pharmaceutics. 2022 Oct 13;14(10):2181. doi: 10.3390/pharmaceutics14102181. Pharmaceutics. 2022. PMID: 36297616 Free PMC article. Review. - Glucosinolate-Degradation Products as Co-Adjuvant Therapy on Prostate Cancer in Vitro.
Núñez-Iglesias MJ, Novío S, García C, Pérez-Muñuzuri E, Soengas P, Cartea E, Velasco P, Freire-Garabal M. Núñez-Iglesias MJ, et al. Int J Mol Sci. 2019 Oct 9;20(20):4977. doi: 10.3390/ijms20204977. Int J Mol Sci. 2019. PMID: 31600887 Free PMC article. - Structural basis for the modulation of MRP2 activity by phosphorylation and drugs.
Mazza T, Roumeliotis TI, Garitta E, Drew D, Rashid ST, Indiveri C, Choudhary JS, Linton KJ, Beis K. Mazza T, et al. Nat Commun. 2024 Mar 4;15(1):1983. doi: 10.1038/s41467-024-46392-8. Nat Commun. 2024. PMID: 38438394 Free PMC article. - Genetic Predictors of Antipsychotic Efflux Impairment via Blood-Brain Barrier: Role of Transport Proteins.
Nasyrova RF, Shnayder NA, Osipova SM, Khasanova AK, Efremov IS, Al-Zamil M, Petrova MM, Narodova EA, Garganeeva NP, Shipulin GA. Nasyrova RF, et al. Genes (Basel). 2023 May 15;14(5):1085. doi: 10.3390/genes14051085. Genes (Basel). 2023. PMID: 37239445 Free PMC article. Review.
References
- Higgins C. F. (1992) ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 - PubMed
- Slot A. J., Molinski S. V., Cole S. P. C. (2011) Mammalian multidrug resistance proteins (MRPs). Essays Biochem. 50, 179–207 - PubMed
- Cole S. P. C., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M. V., Deeley R. G. (1992) Overexpression of a transporter gene in a multidrug resistant human lung cancer cell line. Science 258, 1650–1654 - PubMed
- Cole S. P. C. (2014) Targeting the multidrug resistance protein (MRP1, ABCC1): past, present and future. Annu. Rev. Pharmacol. Toxicol. 54, 95–117 - PubMed
- Szczypka M. S., Wemmie J. A., Moye-Rowley W. S., Thiele D. J. (1994) A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein. J. Biol. Chem. 269, 22853–22857 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous