Expression of insulin-like growth factor 2 receptor in corneal keratocytes during differentiation and in response to wound healing - PubMed (original) (raw)
Expression of insulin-like growth factor 2 receptor in corneal keratocytes during differentiation and in response to wound healing
Richard N Bohnsack et al. Invest Ophthalmol Vis Sci. 2014.
Abstract
Purpose: Insulin-like growth factor 2 receptor (IGF2R) associates with ligands that influence wound healing outcomes. However, the expression pattern of IGF2R and its role in the cornea is unknown.
Methods: Human keratocytes were isolated from donor corneas. Fibroblasts (fibroblast growth factor 2 [FGF2]-treated) or myofibroblasts (TGF-β1-treated) were analyzed for IGF2R and α-smooth muscle actin (α-SMA) expression by Western blotting and immunolocalization. Mouse corneas were wounded in vivo and porcine corneas ex vivo. The IGF2R and α-SMA protein expression were visualized and quantified by immunohistochemistry. The IGF2R gene expression in human corneal fibroblasts was knocked-down with targeted lentiviral shRNA.
Results: The IGF2R is expressed in epithelial and stromal cells of normal human, mouse, and porcine corneas. The IGF2R increases (11.2 ± 0.4-fold) in the epithelial and (11.7 ± 0.9-fold) stromal layers of in vivo wounded mouse corneas. Double-staining with α-SMA- and IGF2R-specific antibodies reveals that IGF2R protein expression is increased in stromal myofibroblasts in the wounded cornea relative to keratocytes in the normal cornea (11.2 ± 0.8-fold). Human primary stromal keratocytes incubated with FGF2 or TGF-β1 in vitro demonstrate increased expression (2.0 ± 0.4-fold) of IGF2R in myofibroblasts relative to fibroblasts. Conversion of IGF2R shRNA-lentiviral particle transduced corneal fibroblasts to myofibroblasts reveals a dependence on IGF2R expression, as only 40% ± 10% of cells transduced converted to myofibroblasts compared to 86% ± 3% in control cells.
Conclusions: The IGF2R protein expression is increased during corneal wound healing and IGF2R regulates human corneal fibroblast to myofibroblast differentiation.
Keywords: IGF2R; TGF-β1; cornea; myofibroblasts; wound healing.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures
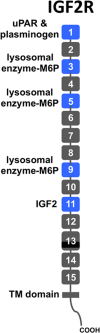
Figure 1
Schematic diagram of IGF2R protein. The IGF2R protein is a type I integral membrane protein with a single transmembrane (TM) domain. It contains 15 contiguous domains (gray and blue rectangles) in its extracellular region. Each domain is approximately 150 residues in length, except domain 13 is larger and contains a 48-residue fibronectin type II (FnII) insert (black). The domains are joined together by a short linker of 5 to 12 residues in length whereas the 15th domain is joined to the transmembrane region by a 25-residue linker. The locations of known ligand binding sites are shown (blue rectangles).
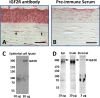
Figure 2
Immunodetection of IGF2R in tissue sections from human cornea. Normal human donor corneas (A, B), were formalin-fixed, paraffin-embedded, and sections incubated with IGF2R-specific polyclonal antibody (A) or the corresponding pre-immune serum (B). Biotinylated secondary antibody, avidin alkaline phosphatase reagent and a fluorescent Vector Red alkaline phosphatase substrate were used for detection of the primary antibody. Scale bar: 40 μm. Arrows indicate representative cells in the stroma with intense staining for IGF2R. (C) Cultured primary human epithelial cell lysates were subjected to Western blot analysis and the membranes probed with IGF2R-specific antibody. The amount of total protein from the cell lysate loaded in each lane is indicated. (D) Lysates from human corneal epithelial, stromal, and endothelial cell layers were subjected to Western blot analysis and the membranes probed with IGF2R-specific antibody. The amount of total protein from the cell lysate loaded in each lane is indicated. All bands below the 300 kDa IGF2R-labeled bands in (C, D) are nonspecific and appear in blots using the preimmune serum.
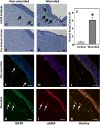
Figure 3
Immunodetection of IGF2R in tissue sections from wounded mouse cornea. Normal mouse corneas (A, B) or mouse corneas subjected to a 2-mm central scraped wound limited to the epithelial layer (C, D) were formalin-fixed, paraffin-embedded, and sections incubated with IGF2R-specific polyclonal antibody (A, C) or the corresponding preimmune serum (B, D). Biotinylated secondary antibody, avidin alkaline phosphatase reagent, and a fluorescent Vector Red alkaline phosphatase substrate were used for detection of the primary antibody. A representative experiment is shown of three independent replicates. Mouse corneas represent the unwounded central cornea (A, B) and the center of the wound bed (C, D). Scale bar: 40 μm. Arrows indicate representative cells in the stroma with intense staining for IGF2R. (E) Quantification of IGF2R staining area in the epithelial and stromal layers of control and wounded mouse samples is shown. Stained areas were above the threshold set based on the preimmune staining. The values represent the mean ± SE. *Indicates 1-tailed Student's _t_-test, P < 0.005.
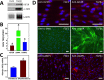
Figure 4
Immunodetection of IGF2R in organ cultures of normal or trephine-wounded porcine corneas. Untreated porcine corneas (A, B, F, H, J) or those wounded by removal of 5 mm trephined portion of the cornea, including epithelium and the anterior stroma (C, D, G, I, K), were mounted on a base containing agarose and collagen as described. The corneas were cultured for 2 weeks, fixed, and sections were obtained. (A–D) Sections were incubated with IGF2R-specific polyclonal antibody (A, C) or the corresponding preimmune serum (B, D) followed by HRP-conjugated goat anti-rabbit antibody and colorimetric DAB substrate. Sections were counterstained with hematoxylin. A representative experiment is shown of three independent replicates. Arrows in (C) indicate regions of the stroma with intense staining for IGF2R. Quantification of IGF2R staining area in the control and wounded samples is shown in (E), and the values represent the mean ± SE. A 1-tailed Student's _t_-test, P < 0.001. (F–K) Immunofluorescence imaging was performed by double-labeling with antibodies specific for IGF2R ([F, G], green, Alexa Fluor488) and α-SMA ([H, I], red, Cy5), and nuclei are stained with DAPI (blue). Overlay of images is shown in (J, K). A representative experiment is shown of three independent replicates. Arrows in (G, I, K) highlight representative myofibroblasts that stain positive for both IGF2R and α-SMA. Scale bar: 100 μm.
Figure 5
Comparison of IGF2R, α-SMA, and LAMP1 levels in myofibroblasts to fibroblasts. (A) Lysates generated from human donor corneal keratocytes cultured in serum-free medium supplemented with 10 ng/mL FGF2 or 1 ng/mL TGF-β1 for 7 days were subjected to Western blot analysis. Extracts of equivalent cell numbers were loaded into each lane. Membranes were probed with antibodies specific to IGF2R, α-SMA, or LAMP1. (B) Quantification of (A) is shown which represents an analysis of corneal keratocyte lysates obtained from independent donors (n = 6, IGF2R; n = 6, α-SMA; n = 5, LAMP1). Values represent the mean ± SE. A 1-sample Student's _t_-test with a hypothetical population mean = 1.0, P < 0.05. (C) Real-time PCR analyses of IGF2R mRNA. (C) Total RNA obtained from cultured fibroblasts and myofibroblasts derived from four independent human donor corneas was analyzed. The relative quantity of IGF2R was compared to the relative quantity of GAPDH, which was used as an internal control. Replicates were performed in triplicate. Values represent the mean ± SE. Student's _t_-test P < 0.001. (D) Human donor corneal keratocytes cultured in 10 ng/mL FGF2 or 1 ng/mL TGF-β1 for 7 days were fixed, incubated with IGF2R-specific antibodies and visualized using a chicken anti-rabbit secondary antibody conjugated to Alexa Fluor 594 (red), FITC-conjugated antibody specific to α-SMA (green), or LAMP1-specific antibodies visualized using a mouse anti-rabbit secondary antibody conjugated to Alexa Fluor 568 (red), and then imaged. Nuclei were stained with Hoescht 33342 dye (blue). Scale bar: 100 μm.
Figure 6
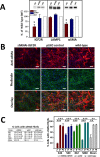
α-SMA fibril formation in primary human corneal myofibroblasts following transduction with control (pLKO) or shRNA-IGF2R lentiviral particles (KD). Lentiviral particle transduced or nontransduced cells (WT) were cultured in 1 ng/mL TGF-β1 for 7 days under serum-free conditions. (A) Lysates were generated and subjected to Western blot analysis (equivalent cell numbers loaded into each lane). Membranes were probed with IGF2R-, α-SMA-, or LAMP1-specific antibodies. Quantification is shown below the representative blots and is derived from corneal cultures obtained from four independent donors. Data are plotted as a percentage of the intensity of IGF2R, α-SMA, or LAMP1 bands in the shRNAi-IGF2R KD cells and cells transduced with pLKO control vector relative to nontransduced WT cells set to 100%. Values represent the mean ± SE. The Student's _t_-test was used to compare the IGF2R KD cells to the pLKO control cells. *P < 0.02, n = 4. (B) Fixed cells were incubated with antibody specific to α-SMA that was visualized using a goat anti-mouse secondary antibody conjugated to Alexa Fluor 568 (red) and FITC-conjugated phalloidin (green). Nuclei were stained with Hoescht 33342 dye. An overlay of the phalloidin and α-SMA–stained cells is shown in the bottom. Representative micrographs are shown. Scale bar: 100 μm. (C) The images from the studies shown in (D) were quantified for the number of cells containing α-SMA fibrils. Approximately 200 to 500 cells per micrograph were scored for the presence of α-SMA–containing fibrils. The percentages shown are derived from four independent donors and are the average for the indicated number (n) of individual micrographs. The mean of the data derived from the four independent human donors also is shown. Values represent the mean ± SE. ANOVA, P < 0.001, individual comparisons use the Holm-Sidak method, **P = 0.002, ***P < 0.001.
Similar articles
- Transforming growth factor beta-3 localization in the corneal response to epithelial-stromal injury and effects on corneal fibroblast transition to myofibroblasts.
Shiju TM, Sampaio LP, Martinez VV, Hilgert GSL, Wilson SE. Shiju TM, et al. Exp Eye Res. 2023 Oct;235:109631. doi: 10.1016/j.exer.2023.109631. Epub 2023 Aug 25. Exp Eye Res. 2023. PMID: 37633325 - Involvement of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in corneal fibroblasts during corneal wound healing.
Izumi K, Kurosaka D, Iwata T, Oguchi Y, Tanaka Y, Mashima Y, Tsubota K. Izumi K, et al. Invest Ophthalmol Vis Sci. 2006 Feb;47(2):591-8. doi: 10.1167/iovs.05-0097. Invest Ophthalmol Vis Sci. 2006. PMID: 16431955 - Transforming growth factor β and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro.
Singh V, Barbosa FL, Torricelli AA, Santhiago MR, Wilson SE. Singh V, et al. Exp Eye Res. 2014 Mar;120:152-60. doi: 10.1016/j.exer.2014.01.003. Epub 2014 Jan 12. Exp Eye Res. 2014. PMID: 24429028 Free PMC article. - The corneal fibrosis response to epithelial-stromal injury.
Torricelli AA, Santhanam A, Wu J, Singh V, Wilson SE. Torricelli AA, et al. Exp Eye Res. 2016 Jan;142:110-8. doi: 10.1016/j.exer.2014.09.012. Exp Eye Res. 2016. PMID: 26675407 Free PMC article. Review. - Corneal wound healing.
Wilson SE. Wilson SE. Exp Eye Res. 2020 Aug;197:108089. doi: 10.1016/j.exer.2020.108089. Epub 2020 Jun 15. Exp Eye Res. 2020. PMID: 32553485 Free PMC article. Review.
Cited by
- Corneal myofibroblasts and fibrosis.
Wilson SE. Wilson SE. Exp Eye Res. 2020 Dec;201:108272. doi: 10.1016/j.exer.2020.108272. Epub 2020 Sep 30. Exp Eye Res. 2020. PMID: 33010289 Free PMC article. Review. - Circadian rhythms, refractive development, and myopia.
Chakraborty R, Ostrin LA, Nickla DL, Iuvone PM, Pardue MT, Stone RA. Chakraborty R, et al. Ophthalmic Physiol Opt. 2018 May;38(3):217-245. doi: 10.1111/opo.12453. Ophthalmic Physiol Opt. 2018. PMID: 29691928 Free PMC article. Review. - Circulating exosomal miR-16-5p and let-7e-5p are associated with bladder fibrosis of diabetic cystopathy.
Xue B, Kadeerhan G, Sun LB, Chen YQ, Hu XF, Zhang ZK, Wang DW. Xue B, et al. Sci Rep. 2024 Jan 8;14(1):837. doi: 10.1038/s41598-024-51451-7. Sci Rep. 2024. PMID: 38191820 Free PMC article. - The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease.
Stuard WL, Titone R, Robertson DM. Stuard WL, et al. Front Endocrinol (Lausanne). 2020 Mar 3;11:24. doi: 10.3389/fendo.2020.00024. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32194500 Free PMC article. Review. - Insulin-like Growth Factor-2 (IGF-2) in Fibrosis.
Zhu Y, Chen L, Song B, Cui Z, Chen G, Yu Z, Song B. Zhu Y, et al. Biomolecules. 2022 Oct 25;12(11):1557. doi: 10.3390/biom12111557. Biomolecules. 2022. PMID: 36358907 Free PMC article. Review.
References
- Wilson SE, Mohan RR, Ambrosio R Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res 2001; 20: 625–637. - PubMed
- Wilson SE, Netto M, Ambrosio R Jr. Corneal cells: chatty in development, homeostasis, wound healing, and disease. Am J Ophthalmol. 2003; 136: 530–536. - PubMed
- Maguen E, Zorapapel NC, Zieske JD, et al. Extracellular matrix and matrix metalloproteinase changes in human corneas after complicated laser-assisted in situ keratomileusis (LASIK). Cornea. 2002; 21: 95–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK042667/DK/NIDDK NIH HHS/United States
- T32 GM062754/GM/NIGMS NIH HHS/United States
- R56 DK042667/DK/NIDDK NIH HHS/United States
- EY021152/EY/NEI NIH HHS/United States
- EY01931/EY/NEI NIH HHS/United States
- R01 EY021152/EY/NEI NIH HHS/United States
- DK042667/DK/NIDDK NIH HHS/United States
- P30 EY001931/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous