Induced pluripotent stem cells restore function in a human cell loss model of open-angle glaucoma - PubMed (original) (raw)
Induced pluripotent stem cells restore function in a human cell loss model of open-angle glaucoma
Diala W Abu-Hassan et al. Stem Cells. 2015 Mar.
Abstract
Normally, trabecular meshwork (TM) and Schlemm's canal inner wall endothelial cells within the aqueous humor outflow pathway maintain intraocular pressure within a narrow safe range. Elevation in intraocular pressure, because of the loss of homeostatic regulation by these outflow pathway cells, is the primary risk factor for vision loss due to glaucomatous optic neuropathy. A notable feature associated with glaucoma is outflow pathway cell loss. Using controlled cell loss in ex vivo perfused human outflow pathway organ culture, we developed compelling experimental evidence that this level of cell loss compromises intraocular pressure homeostatic function. This function was restored by repopulation of the model with fresh TM cells. We then differentiated induced pluripotent stem cells (iPSCs) and used them to repopulate this cell depletion model. These differentiated cells (TM-like iPSCs) became similar to TM cells in both morphology and expression patterns. When transplanted, they were able to fully restore intraocular pressure homeostatic function. This successful transplantation of TM-like iPSCs establishes the conceptual feasibility of using autologous stem cells to restore intraocular pressure regulatory function in open-angle glaucoma patients, providing a novel alternative treatment option.
Keywords: Autologous stem cell transplantation; Cell transplantation; Experimental models; Induced pluripotent stem cells; Somatic stem cells; Stem cell transplantation; Tissue regeneration; Transplantation.
© 2014 The Authors. STEM CELLS Published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures
Figure 1
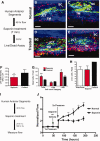
Saponin treatment of human anterior segments. (A): Experimental schematic for cell death assessment after a 7-minute treatment with 0.01% saponin. Frontal sections of normal vehicle-treated control aged anterior segments (B, C) compared to saponin-treated anterior segments (D, E) showing live cells (green) and nuclei of dead cells (red). White scale bars represent 100 µm. (F): Live cell values expressed as GFU per µm field volume, using total area of green fluorescence as a proxy and comparing saponin treated with control. Significance at p < .05 is indicated by *. (G): Dead cell count of red nuclei per three-dimensional (3D) field; both total and broken down into fields showing results for less than or more than 100 nuclei. Significance is indicated as **, p < .001 and *, p < .05. (H): Live cells/field showing comparisons of live cells for glaucoma eyes, saponin-treated, and normal control groups, all normalized to common 3D field volumes. (I): Schematic showing 0.01% saponin treatment pattern for flow studies. (J): Normalized flow rate for perfused human anterior segments treated with saponin or vehicle (normal) and then subjected to intraocular pressure homeostatic 2× pressure challenge. Mean and SEM are shown where n = 8 for normal and n = 17 for saponin-treated anterior segments with significance determined by one-way ANOVA at p < .001 indicated by **. Abbreviations: GFU, green fluorescence units; JCT, juxtacanalicular region; SC, Schlemm's canal; TM, trabecular meshwork.
Figure 2
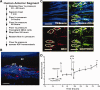
Replacement of saponin-depleted cells with human cultured TM cells. (A): Detailed schematic of treatment protocol. (B): Frontal section showing penetration of QDot (red) labeled HTM cells to all levels of the TM after transplantation. Scale bar is 100 µm. (C): TM beams showing blue autofluorescence from collagen and elastic fibers (TM Beams), CD44 immunohistochemistry (green) to label cell surfaces, and QDot (red) labeled transplanted TM cells. White dashes enclose individual cells, which contain QDots indicating that they were transplanted. Dashes were drawn based on Z-stack three-dimensional scans to identify individual QDot-labeled cells. The scale bar is 100 µm. (D): Transplanted replacement HTM cells (added at the time indicated) restored the intraocular pressure homeostatic response to 2× pressure elevation, which had been compromised by saponin treatment. Line shows mean for six experiments using separate anterior segments and error bars represent the SEM with significance by one-way ANOVA where *, p < .05 and **, p < .001. Abbreviations: HTM, human trabecular meshwork; JCT, juxtacanalicular region; SC, Schlemm's canal; TM, trabecular meshwork.
Figure 3
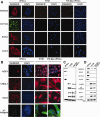
Biomarker expression comparison between iPSCs, TM, and differentiated TM-like iPSCs. (A): Immunohistochemical comparison of levels of four stem cell markers, NANOG, OCT3/4, SOX2, and KLF4 by these three cell types. Cell nuclei are stained with DAPI (blue). All panels are exactly the same size with scale bar = 100 µm. (B): TM cell markers as expressed by iPSCs, HTM, and TM-like iPSC. DAPI nuclear stain is blue, all panels are the exact same size and scales are identical with the white scale bars = 100 µm. (C): Western immunoblot showing levels of these two groups of proteins and (d) gels from quantitative RT-PCR analysis showing levels of mRNA expression for these genes. Loading controls are α-tubulin for Western immunoblots and 18S ribosomal subunit for mRNA gels. Abbreviations: HTM, human trabecular meshwork; iPSCs, induced pluripotent stem cells; TM, trabecular meshwork.
Figure 4
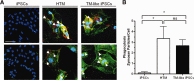
Differentiated TM-like iPSCs can perform phagocytosis, a typical TM cell property. (A): iPSCs, HTM, and differentiated TM-like iPSCs cultured on chamber slides were incubated with fluorescent-labeled zymosan particles for an hour. They were then washed, fixed, and immunostained with LAMP1, a lysosomal marker, to verify internalization. The colocalization (yellow; examples marked with white arrowheads) of zymosan particles (green) with LAMP1 (red) indicates the phagocytosis of the particles. Both HTM and TM-like iPSCs phagocytosed the particles but iPSCs did not. Nuclei were demarcated by DAPI staining (blue). Scale bar represents 100 µm. (B): The number of zymosan particles that colocalize with LAMP1 was counted and the total number was divided by total number of cells in the field. Significance is indicated by * where p < .05 and ns indicates not significant. Abbreviations: HTM, human trabecular meshwork; iPSCs, induced pluripotent stem cells; TM, trabecular meshwork.
Figure 5
Replacement of saponin-depleted cells with TM-like iPSCs in anterior segments. (A): Confocal analysis of frontal section showing transplanted QDot (red) labeled TM-like iPSCs at all levels of the outflow pathway. Blue shows TM beam collagen and elastic fiber autofluorescence; scale bars are 100 µm. (B): Frontal section after perfusion protocol shows TM beams (blue), cell surface CD44 immunostaining (green), and QDot-labeled transplanted TM-like iPSCs (red). Scale bar indicates 100 µm. White dashes outline individual transplanted cells attached to TM beams. Outlines were determined by scanning through the three-dimensional confocal Z-stacks. (C): After 1× perfusion, saponin was added as indicated, rinsed out, and perfusion resumed for 24 hours at 1× pressure. The 2× pressure challenge gave no intraocular pressure (IOP) homeostatic response. TM-like iPSCs were added and allowed to attach for 24 hours; flow was resumed at 1× pressure and then increased to 2× pressure. A typical IOP homeostatic response now occurred over several days. n = 6 experiments with separate anterior segments and significance by one-way ANOVA is *, p < .05 and **, p < .001. (D): Similar experiment with 300,000 DF transplanted as a control. They actually triggered a reduction in outflow but no IOP response to 2× pressure challenge. (E): Effects of mock-differentiated iPSC EB, which had been exposed only to 5% aqueous humor during a parallel differentiation period, (F) HUVECs, or (G) no cells added at all were compared. Only differentiated TM-like iPSCs produced an IOP homeostatic pressure response. Abbreviations: DF, dermal fibroblasts; EB, embryoid bodies; HTM, human trabecular meshwork; HUVEC, human umbilical vein endothelial cell; iPSCs, induced pluripotent stem cells; TM, trabecular meshwork.
Similar articles
- iPSC-Derived Trabecular Meshwork Cells Stimulate Endogenous TM Cell Division Through Gap Junction in a Mouse Model of Glaucoma.
Sui S, Yu H, Wang X, Wang W, Yang X, Pan X, Zhou Q, Xin C, Du R, Wu S, Zhang J, Cao Q, Wang N, Kuehn MH, Zhu W. Sui S, et al. Invest Ophthalmol Vis Sci. 2021 Aug 2;62(10):28. doi: 10.1167/iovs.62.10.28. Invest Ophthalmol Vis Sci. 2021. PMID: 34427623 Free PMC article. - iPSCs-Based Therapy for Trabecular Meshwork.
Zhu W, Zhang X, Wu S, Wang N, Kuehn MH. Zhu W, et al. Handb Exp Pharmacol. 2023;281:277-300. doi: 10.1007/164_2023_671. Handb Exp Pharmacol. 2023. PMID: 37495850 - Restoration of Aqueous Humor Outflow Following Transplantation of iPSC-Derived Trabecular Meshwork Cells in a Transgenic Mouse Model of Glaucoma.
Zhu W, Jain A, Gramlich OW, Tucker BA, Sheffield VC, Kuehn MH. Zhu W, et al. Invest Ophthalmol Vis Sci. 2017 Apr 1;58(4):2054-2062. doi: 10.1167/iovs.16-20672. Invest Ophthalmol Vis Sci. 2017. PMID: 28384726 Free PMC article. - Cell-Based Therapies for Trabecular Meshwork Regeneration to Treat Glaucoma.
Mallick S, Sharma M, Kumar A, Du Y. Mallick S, et al. Biomolecules. 2021 Aug 24;11(9):1258. doi: 10.3390/biom11091258. Biomolecules. 2021. PMID: 34572471 Free PMC article. Review. - Replacement of the Trabecular Meshwork Cells-A Way Ahead in IOP Control?
Fan X, Bilir EK, Kingston OA, Oldershaw RA, Kearns VR, Willoughby CE, Sheridan CM. Fan X, et al. Biomolecules. 2021 Sep 16;11(9):1371. doi: 10.3390/biom11091371. Biomolecules. 2021. PMID: 34572584 Free PMC article. Review.
Cited by
- Two-step induction of trabecular meshwork cells from induced pluripotent stem cells for glaucoma.
Kumar A, Cheng T, Song W, Cheuk B, Yang E, Yang L, Xie Y, Du Y. Kumar A, et al. Biochem Biophys Res Commun. 2020 Aug 20;529(2):411-417. doi: 10.1016/j.bbrc.2020.05.225. Epub 2020 Jul 1. Biochem Biophys Res Commun. 2020. PMID: 32703444 Free PMC article. - Evidence of Müller Glia Conversion Into Retina Ganglion Cells Using Neurogenin2.
Guimarães RPM, Landeira BS, Coelho DM, Golbert DCF, Silveira MS, Linden R, de Melo Reis RA, Costa MR. Guimarães RPM, et al. Front Cell Neurosci. 2018 Nov 12;12:410. doi: 10.3389/fncel.2018.00410. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30483060 Free PMC article. - Regenerating Eye Tissues to Preserve and Restore Vision.
Stern JH, Tian Y, Funderburgh J, Pellegrini G, Zhang K, Goldberg JL, Ali RR, Young M, Xie Y, Temple S. Stern JH, et al. Cell Stem Cell. 2018 Jun 1;22(6):834-849. doi: 10.1016/j.stem.2018.05.013. Cell Stem Cell. 2018. PMID: 29859174 Free PMC article. Review. - Gap junction connexin43 is a key element in mediating phagocytosis activity in human trabecular meshwork cells.
Li X, Nagy JI, Li D, Acott TS, Kelley MJ. Li X, et al. Int J Physiol Pathophysiol Pharmacol. 2020 Feb 25;12(1):25-31. eCollection 2020. Int J Physiol Pathophysiol Pharmacol. 2020. PMID: 32211119 Free PMC article. - Regenerative treatment of ophthalmic diseases with stem cells: Principles, progress, and challenges.
Niu Y, Ji J, Yao K, Fu Q. Niu Y, et al. Adv Ophthalmol Pract Res. 2024 Feb 28;4(2):52-64. doi: 10.1016/j.aopr.2024.02.001. eCollection 2024 May-Jun. Adv Ophthalmol Pract Res. 2024. PMID: 38586868 Free PMC article. Review.
References
- Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. - PubMed
- Morrison JC, Pollack IP, editors. Glaucoma: Science and Practice. New York, Stuttgart: Thieme Medical Publishers; 2003. pp. 34–41.
- Tamm ER. The trabecular meshwork outflow pathways: Structural and functional aspects. Exp Eye Res. 2009;88:648–655. - PubMed
Publication types
MeSH terms
Grants and funding
- EY010572/EY/NEI NIH HHS/United States
- EY003279/EY/NEI NIH HHS/United States
- P30 EY010572/EY/NEI NIH HHS/United States
- R01 EY003279/EY/NEI NIH HHS/United States
- EY021800/EY/NEI NIH HHS/United States
- EY008247/EY/NEI NIH HHS/United States
- R01 EY025721/EY/NEI NIH HHS/United States
- R01 EY008247/EY/NEI NIH HHS/United States
- R01 EY021800/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources