An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides - PubMed (original) (raw)
Review
doi: 10.2147/DDDT.S72892. eCollection 2015.
Zhi-Wei Zhou 2, Hui-Ping Sheng 3, Lan-Jie He 4, Xue-Wen Fan 5, Zhi-Xu He 6, Tao Sun 7, Xueji Zhang 8, Ruan Jin Zhao 9, Ling Gu 10, Chuanhai Cao 2, Shu-Feng Zhou 11
Affiliations
- PMID: 25552899
- PMCID: PMC4277126
- DOI: 10.2147/DDDT.S72892
Review
An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides
Jiang Cheng et al. Drug Des Devel Ther. 2014.
Abstract
Lycium barbarum berries, also named wolfberry, Fructus lycii, and Goji berries, have been used in the People's Republic of China and other Asian countries for more than 2,000 years as a traditional medicinal herb and food supplement. L. barbarum polysaccharides (LBPs) are the primary active components of L. barbarum berries and have been reported to possess a wide array of pharmacological activities. Herein, we update our knowledge on the main pharmacological activities and possible molecular targets of LBPs. Several clinical studies in healthy subjects show that consumption of wolfberry juice improves general wellbeing and immune functions. LBPs are reported to have antioxidative and antiaging properties in different models. LBPs show antitumor activities against various types of cancer cells and inhibit tumor growth in nude mice through induction of apoptosis and cell cycle arrest. LBPs may potentiate the efficacy of lymphokine activated killer/interleukin-2 combination therapy in cancer patients. LBPs exhibit significant hypoglycemic effects and insulin-sensitizing activity by increasing glucose metabolism and insulin secretion and promoting pancreatic β-cell proliferation. They protect retinal ganglion cells in experimental models of glaucoma. LBPs protect the liver from injuries due to exposure to toxic chemicals or other insults. They also show potent immunoenhancing activities in vitro and in vivo. Furthermore, LBPs protect against neuronal injury and loss induced by β-amyloid peptide, glutamate excitotoxicity, ischemic/reperfusion, and other neurotoxic insults. LBPs ameliorate the symptoms of mice with Alzheimer's disease and enhance neurogenesis in the hippocampus and subventricular zone, improving learning and memory abilities. They reduce irradiation- or chemotherapy-induced organ toxicities. LBPs are beneficial to male reproduction by increasing the quality, quantity, and motility of sperm, improving sexual performance, and protecting the testis against toxic insults. Moreover, LBPs exhibit hypolipidemic, cardioprotective, antiviral, and antiinflammatory activities. There is increasing evidence from preclinical and clinical studies supporting the therapeutic and health-promoting effects of LBPs, but further mechanistic and clinical studies are warranted to establish the dose-response relationships and safety profiles of LBPs.
Keywords: T cell; anti-aging; antioxidant; apoptosis; cancer; chemotherapy; ischemic/reperfusion injury; mechanism; natural killer.
Figures
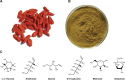
Figure 1
Lycium barbarum fruits (A), brown-colored LBPs (B), and six main monosaccharaides present in LBPs (C). Abbreviation: LBPs, L. barbarum polysaccharides.
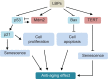
Figure 2
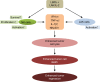
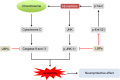
Possible mechanisms for the anti-aging effect of LBPs in zebrafish. Notes: LBPs show marked anti-aging effect through the inhibition of cell apoptosis and senescence. LBPs decrease the expression of p53, p21, and Bax; whereas increase the expression of Mdm2 and TERT in zebrafish. During aging, p53 is activated, triggering expression of pro-senescence targets such as p21, responsible for G1 cell-cycle arrest and E2F7, pivotal in repression of mitotic genes. Mdm2 acts both as an E3 ubiquitin ligase that recognizes the N-terminal trans-activation domain of p53 and as an inhibitor of p53 transcriptional activation. Abbreviations: LBPs, Lycium barbarum polysaccharides; TERT, telomerase reverse trans criptase.
Figure 3
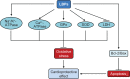
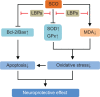
Possible mechanisms for the antioxidant activities of LBPs. Notes: LBPs increase SOD, GPx, CAT, and GR activities, thereby inhibiting oxidative stress-induced damage. LBPs ameliorate oxidative stress-induced cellular apoptosis. LBPs can delay angiotensin II-induced aging of HUVECs by downregulating the expression of p53 and p16. In the I/R heart, LBPs significantly decrease the myocardium LDH level, increase Na+/K+-ATPase and Ca2+-ATPase activities. LBPs ameliorate oxidative stress-induced cellular apoptosis by downregulating Bax and upregulating Bcl-2. Abbreviations: LBPs, Lycium barbarum polysaccharides; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; GR, glutathione reductase; I/R, ischemia/reperfusion; HUVECs, human umbilical vein endothelial cells; Nrf2, nuclear factor erythroid 2-related factor; ROS, reactive oxygen species; MDA, malondialdehyde.
Figure 4
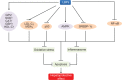
Possible mechanisms for the anticancer activities of LBPs. Notes: LBPs inhibit the proliferation of various types of cancer cells and induce cell cycle arrest at the G0/G1, S, or G2/M phase. LBPs inhibit the growth of cancer xenografts in nude mice. In cancer patients, LAK/IL-2 plus LBP treatment leads to more marked increase in NK and LAK cell activity than LAK/IL-2 alone. LBPs regulate the expression of Bcl-2 and Bax to induce tumor cell apoptosis via increasing intracellular Ca2+ concentration and mitochondrial pathway. LBPs inhibit the growth of MCF-7 cells through activation of Erk1/2 and modulation of estrogen metabolism. LBPs downregulate the expression of cyclin D, cyclin E, and CDK2 in colon cancer cells. LBPs stimulate p53-mediated apoptosis in liver cancer cells due to inhibition of NF-κB. Abbreviations: LBPs, Lycium barbarum polysaccharides; IL-2, interleukin-2; NK, natural killer; LAK, lymphokine activated killer; MCF-7, Michigan Cancer Foundation-7; CDK2, cyclin-dependent kinase 2; NF-κB, nuclear factor κB.
Figure 5
LBPs potentiate the immune-enhancing activity of LAK/IL-2 therapy in cancer patients. Notes: LBPs enhance NK and LAK cell activities in cancer patients treated with LAK/IL-2, resulting in an increase in tumor cell lysis and death. Abbreviations: LBPs, Lycium barbarum polysaccharides; IFN, interferon; IL-2, interleukin-2; NK, natural killer; LAK, lymphokine activated killer; TNF, tumor necrosis factor.
Figure 6
Possible mechanisms for the cardioprotective effects of LBPs. Notes: LBPs exert a remarkable cardioprotective effect in in vitro and in vivo models. LBPs increase the activity of Na+/K+-ATPase and Ca2+-ATPase, enhance the expression of GPx, SOD, and reduce the production of LDH, resulting in a marked reduction in oxidative stress. Also, LBPs increase the ratio of anti-apoptotic factor (Bcl-2) and decrease the pro-apoptotic factor (Bax), protecting the myocardial cells from apoptotic cell death. Abbreviations: LBPs, Lycium barbarum polysaccharides; SOD, superoxide dismutase; GPx, glutathione peroxidase; LDH, lactate dehydrogenase.
Figure 7
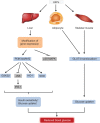
Possible mechanisms for the hepatoprotective effects of LBPs. Notes: LBPs showed significant hepatoprotective effect in in vivo models via suppression of oxidative stress, inflammatory response, and apoptosis. LBPs increase the levels and activities of GPx, SOD, CAT, GSH, HDL-C, and AMPK, but reduce the levels and activities of LDL-C, MDA, p53, SREBP-1c, and NF-κB in vivo. Abbreviations: LBPs, Lycium barbarum polysaccharides; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; GSH, glutathione; HDL-C, high-density lipoprotein cholesterol; AMPK, monophosphate-activated protein kinase; LDL-C, low-density lipoprotein cholesterol; MDA, malondialdehyde; SREBP-1c, sterol regulatory element-binding protein-1c; NF-κB, nuclear factor κB.
Figure 8
Possible mechanisms for the hypoglycemic effects of LBPs. Notes: LBPs significantly promote glucose uptake involving several signaling pathways in the liver and muscle. LBPs increase the content of GLUT4 and promote the translocation of GLUT4 from cytosol to cell membrane, enhancing glucose uptake in Wistar rats muscle. LBPs also increase the phosphorylation of PI3K/Akt/Nrf2 and repress the activation of JNK, promoting insulin-signaling pathway and insulin-dependent glucose uptake in C57BL/6J mice liver. Furthermore, LBPs activate PI3K- and p38MAPK-mediated signaling pathway, improving insulin sensitivity in rats. Moreover, consumption of GoChi, a standardized Goji juice containing 13.6 mg/mL LBPs promotes caloric expenditure and reduces waist circumference in healthy subjects. Abbreviations: LBPs, Lycium barbarum polysaccharides; JNK, Jun N-terminal kinases; Nrf2, nuclear factor erythroid 2-related factor; PI3K, phosphatidylinositol 3-kinase; p38 MAPK, p38 mitogen activated protein kinase; GLUT4, glucose transporter type-4; IRS-1, insulin receptor substrate-1; HO-1, heme oxygenase-1; SOD, superoxide dismutase; GSK3β, glycogen synthase kinase 3β.
Figure 9
Possible mechanisms for the immunomodulating effects of LBPs. Notes: LBPs have been found to have a variety of immune-modulatory activities in vitro and in vivo. LBPs promote the proliferation and activity of splenocytes, T cells, B cells, macrophages and NK cells. LBPs induce IL-6, IL-8, IL-10, and TNF-α production in splenocytes. LBPs stimulate PBMCs to produce IL-2 and TNF-α. IL-2 stimulates growth and differentiation of T cells. LBPs promote T lymphocytes and macrophages to release important cytokines such as IL-10 and TNF-α. IL-10 inhibits the production of IFN-γ, IL-2, IL-3, TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) by activated macrophages and by helper T cells. LBPs activate macrophages and upregulate the expressions of CD40, CD80, CD86, and MHC-II molecules. LBPs activate transcription factors NF-κB and AP-1, induce TNF-α, IL-1β, and IL-12p40 expression in macrophages. LBPs significantly enhance macrophage endocytic and phagocytic capacities. LBPs promote the cytotoxicity of NK cells by enhancing IFN-γ and perforin release and the expression of the activating receptors NKp30 and NKG2D. LBPs also stimulate macrophages and NK cells to release TNF-α and IL-1β. LBPs activate the transcription factors NFAT and AP-1 and prompt CD25 (IL-2 receptor-α) expression. LBPs induce the maturation of DCs and improve their antigen-presenting function. LBPs can upregulate the expression of CD40, CD80, CD86, and MHC-II molecules in bone marrow- and peripheral blood-derived DCs, downregulate DC uptake of Ag, enhance allostimulatory activity of DCs, and induce the production of IL-12p40 and p70 in DCs. IL-12 is involved in the stimulation and maintenance of Th1 cellular immune responses and also has an important role in enhancing the cytotoxic function of NKs. LBP-treated DCs can enhance both Th1 and Th2 responses. LBPs potentiate the immune responses of DNA vaccine against Chlamydophila abortus in mice. LBPs also activate CXCR5+PD-1+ Tfh cells and induce IL-21 secretion. Dietary wolfberry supplementation enhances both in vivo and ex vivo T-cell response to specific antigens. Elderly persons who consume Lacto-Wolfberry for 3 months show higher serum influenza-specific IgG concentrations and seroconversion rate after receiving an influenza vaccine. Abbreviations: Ag, antigen; AP-1, activator protein-1; GM-CSF, granulocyte-macrophage colony-stimulating factor; DCs, dendritic cells; IFN-γ, interferon-γ; IL, interleukin; LBP, Lycium barbarum polysaccharide; MHC-II, class II major histocompatibility complex; NF-κB, nuclear factor κB; NFAT, nuclear factor of activated T-cells; NK, natural killer; PD, programmed death; TNF, tumor necrosis factor; JNK, Jun N-terminal kinases; Nrf2, nuclear factor erythroid 2-related factor; PI3K, phosphatidylinositol 3-kinase; p38 MAPK, p38 mitogen activated protein kinase; GLUT4, glucose transporter type-4; IRS-1, insulin receptor substrate-1; HO-1, heme oxygenase-1; SOD, superoxide dismutase; GSK3β, glycogen synthase kinase 3β; Tfh, T follicular helper.
Figure 10
Possible mechanisms for the neuroprotective effects of LBPs against MCAO-induced brain injuries. Notes: LBPs treatment protects neurons against MCAO-induced brain injuries mainly via reduction of oxidative stress, inhibition of apoptosis, and increase in the integrity of BBB in mice. LBPs increase the activities of SOD, GPx, CAT, and LDH, but decrease the content of MDA and lipid peroxidation, resulting in a reduction in oxidative stress. LBPs inhibit the expression of cytochrome C, cleave caspase-9, cleaved caspase-3, Bax, and cleaved PARP-1, but increase the expression level of Bcl-2, leading to inhibition of apoptosis. In addition, LBPs increase the expression of occludin but decrease the expression of MMP-9 and aquaporin-4, increasing the integrity of BBB. Abbreviations: LBPs, Lycium barbarum polysaccharides; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; LDH, lactate dehydrogenase; MCAO, middle cerebral artery occlusion; MDA, malondialdehyde; PARP, poly(ADP-ribose) polymerase; MMP-9, matrix metalloproteinase-9; BBB, blood–brain barrier; MHC-II, Class II major histocompatibility complex; TNF, tumor necrosis factor; IL, interleukin; IgG, immunoglobulin G; IFN, interferon; NK, natural killer; Tfh, follicular helper T cell; NKp30, natural killer cell p30-related protein.
Figure 11
Possible mechanisms for the neuroprotective effects of LBPs against Aβ-induced neurotoxicity and Alzheimer’s disease. Notes: LBPs protect neurons against Aβ-induced apoptosis by reducing the activity of both caspase-3 and -2, but not caspase-8 and -9. LBPs inhibit the phosphorylation of JNK-1 at Thr183/Tyr185 and its substrates c-Jun-I at Ser73 and c-Jun-II at Ser63 in neurons. LBPs reduce the phosphorylation of Erk1/2m but not GSK3β. LBPs also markedly reduced Aβ-induced PKR phosphorylation. PKR is an intracellular sensor of stress and can arrest protein synthesis by phosphorylating the alpha subunit of the translation initiation factor eIF2. LBPs also significantly reduce homocysteine-induced phosphorylation of Tau-1 at Ser198/199/202, pS396 at Ser396, and pS214 at Ser214 as well as cleavage of Tau. Abbreviations: Aβ, amyloid-β; LBPs, Lycium barbarum polysaccharides; JNK, Jun N-terminal kinases; GSK3β, glycogen synthase kinase 3β; PKR, protein kinase; eIF2, eukaryotic initiation factor 2.
Figure 12
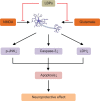
Possible mechanisms for the neuroprotective effects of LBPs against SCO-induced neurotoxicity. Notes: LBPs protect neurons against SCO-induced neurotoxicity through the reduction of the oxidative stress and apoptosis. LBPs increase the activities of SOD and GPx, restore the balance of Bcl-2 to Bax, but decrease the content of MDA. Abbreviations: LBPs, Lycium barbarum polysaccharides; SCO, scopolamine; SOD, superoxide dismutase; GPx, glutathione peroxidase; MDA, malondialdehyde.
Figure 13
Possible mechanisms for the neuroprotective effects of LBPs against glutamate-induced neurotoxicity. Notes: LBPs attenuate glutamate- and NMDA-induced neuronal damage. LBPs decrease the activity of LDH and inhibit the phosphorylation of JNK and the expression of caspase-3, resulting in a decrease in apoptosis. Abbreviations: LBPs, Lycium barbarum polysaccharides; NMDA, _N_-methyl-D-aspartate; LDH, glutathione peroxidase; JNK, Jun N-terminal kinases; p, phosphorylated.
Figure 14
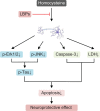
Possible mechanisms for the neuroprotective effects of LBPs against homocysteine-induced neurotoxicity. Notes: LBPs exert neuroprotective effects on cortical neurons exposed to homocysteine via modulation of JNK and Erk1/2 pathways. LBPs suppress the phosphorylation of Erk1/2 and JNK, resulting in an inhibition of phosphorylation of Tau; LBPs also reduce the expression level of caspase-3 and decrease the activity of LDH. Abbreviations: LBPs, Lycium barbarum polysaccharides; LDH, glutathione peroxidase; JNK, Jun N-terminal kinases; Erk1/2, extracellular signal-regulated kinase 1/2; p, phosphorylated.
Figure 15
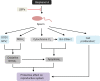
Possible mechanisms for the protective effects of LBPs against bisphenol A-induced sperimatogenic damage. Notes: LBPs exhibit protective effect on then reproductive system via the regulation of oxidative stress, apoptosis, and cell proliferation. LBPs increase the activities of SOD and GPx and restore the balance of Bcl-2 to Bax. LBPs promote cell proliferation but decrease the expression level of cytochrome C and the content of MDA. Abbreviations: LBPs, Lycium barbarum polysaccharides; SOD, superoxide dismutase; GPx, glutathione peroxidase; MDA, malondialdehyde.
Similar articles
- Immune activities of polysaccharides isolated from Lycium barbarum L. What do we know so far?
Xiao Z, Deng Q, Zhou W, Zhang Y. Xiao Z, et al. Pharmacol Ther. 2022 Jan;229:107921. doi: 10.1016/j.pharmthera.2021.107921. Epub 2021 Jun 24. Pharmacol Ther. 2022. PMID: 34174277 Review. - Effects of Polysaccharides in Lycium Barbarum Berries from Different Regions of China on Macrophages Function and their Correlation to the Glycosidic Linkages.
Xie J, Wu DT, Li WZ, Ning CG, Tang YP, Zhao J, Li SP. Xie J, et al. J Food Sci. 2017 Oct;82(10):2411-2420. doi: 10.1111/1750-3841.13813. Epub 2017 Aug 23. J Food Sci. 2017. PMID: 28833151 - A review of Lycium barbarum polysaccharides: Extraction, purification, structural-property relationships, and bioactive molecular mechanisms.
Wang J, Li S, Zhang H, Zhang X. Wang J, et al. Carbohydr Res. 2024 Oct;544:109230. doi: 10.1016/j.carres.2024.109230. Epub 2024 Aug 9. Carbohydr Res. 2024. PMID: 39137472 Review. - [Structure-activity relationship of Lycium barbarum polysaccharides].
Liang XF, Zhang F, Jiang YX, Liu MQ, Guo S, Qian DW, Duan JA. Liang XF, et al. Zhongguo Zhong Yao Za Zhi. 2023 May;48(9):2387-2395. doi: 10.19540/j.cnki.cjcmm.20221219.602. Zhongguo Zhong Yao Za Zhi. 2023. PMID: 37282868 Chinese. - Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity.
Potterat O. Potterat O. Planta Med. 2010 Jan;76(1):7-19. doi: 10.1055/s-0029-1186218. Epub 2009 Oct 20. Planta Med. 2010. PMID: 19844860 Review.
Cited by
- Exploring the Role of Lycium barbarum Polysaccharide in Corneal Injury Repair and Investigating the Relevant Mechanisms through In Vivo and In Vitro Experiments.
Liu Q, Nan Y, Yang Y, Li X, Jiang W, Jiao T, Li J, Jia X, Ye M, Niu Y, Yuan L. Liu Q, et al. Molecules. 2023 Dec 20;29(1):49. doi: 10.3390/molecules29010049. Molecules. 2023. PMID: 38202631 Free PMC article. - Protective effect of Lycium barbarum leaf extracts on atopic dermatitis: in vitro and in vivo studies.
Lee HS, Bae EY, Ly SY. Lee HS, et al. Nutr Res Pract. 2023 Oct;17(5):855-869. doi: 10.4162/nrp.2023.17.5.855. Epub 2023 Jul 11. Nutr Res Pract. 2023. PMID: 37780223 Free PMC article. - In Vitro Protective Effects of Lycium barbarum Berries Cultivated in Umbria (Italy) on Human Hepatocellular Carcinoma Cells.
Ceccarini MR, Vannini S, Cataldi S, Moretti M, Villarini M, Fioretti B, Albi E, Beccari T, Codini M. Ceccarini MR, et al. Biomed Res Int. 2016;2016:7529521. doi: 10.1155/2016/7529521. Epub 2016 Nov 14. Biomed Res Int. 2016. PMID: 27965980 Free PMC article. - Lycium barbarum polysaccharides exert an antioxidative effect on rat chondrocytes by activating the nuclear factor (erythroid-derived 2)-like 2 signaling pathway.
Chen Y, Bi Q, Zhu Z, Zhang S, Xu J, Dou X, Mao W. Chen Y, et al. Arch Med Sci. 2018 Jul 10;16(4):964-973. doi: 10.5114/aoms.2018.77036. eCollection 2020. Arch Med Sci. 2018. PMID: 32542100 Free PMC article. - Black Goji Berry (Lycium ruthenicum Murray): A Review of Its Pharmacological Activity.
Lee HS, Choi CI. Lee HS, et al. Nutrients. 2023 Sep 27;15(19):4181. doi: 10.3390/nu15194181. Nutrients. 2023. PMID: 37836464 Free PMC article. Review.
References
- Potterat O. Goji (Lycium barbarum and L. chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010;76(1):7–19. - PubMed
- Ulbricht C, Bryan JK, Costa D, et al. An evidence-based systematic review of Goji (Lyciumspp.) by the Natural Standard Research Collaboration. J Diet Suppl. 2014 May 7; Epub. - PubMed
- Toyoda-Ono Y, Maeda M, Nakao M, Yoshimura M, Sugiura-Tomimori N, Fukami H. 2-O-(β-D-glucopyranosyl)ascorbic acid, a novel ascorbic acid analogue isolated from Lycium fruit. J Agric Food Chem. 2004;52(7):2092–2096. - PubMed
- Huang LJ, Tian GY, Ji GZ. Structure elucidation of glycan of glycocon-jugate LbGp3 isolated from the fruit of Lycium barbarum L. J Asian Nat Prod Res. 1999;1(4):259–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical