Updated estimates of neural tube defects prevented by mandatory folic Acid fortification - United States, 1995-2011 - PubMed (original) (raw)
- PMID: 25590678
- PMCID: PMC4584791
Updated estimates of neural tube defects prevented by mandatory folic Acid fortification - United States, 1995-2011
Jennifer Williams et al. MMWR Morb Mortal Wkly Rep. 2015.
Abstract
In 1992, the U.S. Public Health Service recommended that all women capable of becoming pregnant consume 400 µg of folic acid daily to prevent neural tube defects (NTDs). NTDs are major birth defects of the brain and spine that occur early in pregnancy as a result of improper closure of the embryonic neural tube, which can lead to death or varying degrees of disability. The two most common NTDs are anencephaly and spina bifida. Beginning in 1998, the United States mandated fortification of enriched cereal grain products with 140 µg of folic acid per 100 g. Immediately after mandatory fortification, the birth prevalence of NTD cases declined. Fortification was estimated to avert approximately 1,000 NTD-affected pregnancies annually. To provide updated estimates of the birth prevalence of NTDs in the period after introduction of mandatory folic acid fortification (i.e., the post-fortification period), data from 19 population-based birth defects surveillance programs in the United States, covering the years 1999-2011, were examined. After the initial decrease, NTD birth prevalence during the post-fortification period has remained relatively stable. The number of births occurring annually without NTDs that would otherwise have been affected is approximately 1,326 (95% confidence interval = 1,122-1,531). Mandatory folic acid fortification remains an effective public health intervention. There remain opportunities for prevention among women with lower folic acid intakes, especially among Hispanic women, to further reduce the prevalence of NTDs in the United States.
Figures
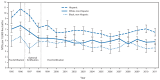
FIGURE
Prevalence of neural tube defects (NTDs) (anencephaly and spina bifida) before and after mandatory folic acid fortification, by maternal race/ethnicity — 19 population-based birth defects surveillance programs,* United States, 1995–2011 * Contributing programs are based in Arkansas, Arizona, California, Colorado, Georgia, Illinois, Iowa, Kentucky, Maryland, New Jersey, New York, North Carolina, Oklahoma, Puerto Rico, South Carolina, Texas, Utah, West Virginia, and Wisconsin. † 95% confidence interval.
Similar articles
- Estimate of the potential impact of folic acid fortification of corn masa flour on the prevention of neural tube defects.
Tinker SC, Devine O, Mai C, Hamner HC, Reefhuis J, Gilboa SM, Dowling NF, Honein MA. Tinker SC, et al. Birth Defects Res A Clin Mol Teratol. 2013 Oct;97(10):649-57. doi: 10.1002/bdra.23158. Birth Defects Res A Clin Mol Teratol. 2013. PMID: 24142499 Free PMC article. - Spina bifida and anencephaly before and after folic acid mandate--United States, 1995-1996 and 1999-2000.
Centers for Disease Control and Prevention (CDC). Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep. 2004 May 7;53(17):362-5. MMWR Morb Mortal Wkly Rep. 2004. PMID: 15129193 - Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995-2002.
Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Williams LJ, et al. Pediatrics. 2005 Sep;116(3):580-6. doi: 10.1542/peds.2005-0592. Pediatrics. 2005. PMID: 16140696 - Status of prevention of neural tube defects post-folic acid fortification of cereal grains in South Africa.
Kancherla V, Randall P, Christianson AL, Malherbe HL. Kancherla V, et al. Public Health Nutr. 2024 Nov 29;27(1):e258. doi: 10.1017/S1368980024002271. Public Health Nutr. 2024. PMID: 39610362 Free PMC article. Review. - Fortification of flour with folic acid.
Berry RJ, Bailey L, Mulinare J, Bower C; Folic Acid Working Group. Berry RJ, et al. Food Nutr Bull. 2010 Mar;31(1 Suppl):S22-35. doi: 10.1177/15648265100311S103. Food Nutr Bull. 2010. PMID: 20629350 Review.
Cited by
- Associations between prenatal exposure to cadmium and lead with neural tube defect risks are modified by single-nucleotide polymorphisms of fetal MTHFR and SOD2: a case-control study.
Liu M, Yu J, Su Z, Sun Y, Liu Y, Xie Q, Li Z, Wang L, Zhang J, Jin L, Ren A. Liu M, et al. Environ Health. 2021 Jun 5;20(1):66. doi: 10.1186/s12940-021-00752-9. Environ Health. 2021. PMID: 34090432 Free PMC article. - Preconception folic acid supplementation for the prevention of birth defects: a prospective, population-based cohort study in mainland China.
Zhou Q, Dong G, Wang Q, Shen H, Zhang Y, Zhang S, Chen J, Li X. Zhou Q, et al. BMC Pregnancy Childbirth. 2024 Feb 6;24(1):114. doi: 10.1186/s12884-024-06283-8. BMC Pregnancy Childbirth. 2024. PMID: 38321376 Free PMC article. - Prevalence and determinants of preconception folic acid use: an Italian multicenter survey.
Nilsen RM, Leoncini E, Gastaldi P, Allegri V, Agostino R, Faravelli F, Ferrazzoli F, Finale E, Ghirri P, Scarano G, Mastroiacovo P. Nilsen RM, et al. Ital J Pediatr. 2016 Jul 13;42(1):65. doi: 10.1186/s13052-016-0278-z. Ital J Pediatr. 2016. PMID: 27411491 Free PMC article. - Classifying by cause and preventing the many causes of spina bifida and anencephaly.
Oakley GP Jr. Oakley GP Jr. Pediatr Res. 2020 Jan;87(2):183-184. doi: 10.1038/s41390-019-0658-3. Epub 2019 Nov 4. Pediatr Res. 2020. PMID: 31683272 No abstract available. - Association between Intake of Total Dairy and Individual Dairy Foods and Markers of Folate, Vitamin B6 and Vitamin B12 Status in the U.S. Population.
Cifelli CJ, Agarwal S, Fulgoni Iii VL. Cifelli CJ, et al. Nutrients. 2022 Jun 13;14(12):2441. doi: 10.3390/nu14122441. Nutrients. 2022. PMID: 35745171 Free PMC article.
References
- CDC. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep. 1992;41(RR-14) - PubMed
- CDC. CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morbid Mortal Wkly Rep. 2010;59:980–4. - PubMed
- CDC. Spina bifida and anencephaly before and after folic acid mandate—United States, 1995–1996 and 1999–2000. MMWR Morbid Mortal Wkly Rep. 2004;53:362–5. - PubMed
- Grosse SD, Ouyang L, Collins JS, Green D, Dean JH, Stevenson RE. Economic evaluation of a neural tube defect recurrence-prevention program. Am J Prev Med. 2008;35:572–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical