GP96 is a GARP chaperone and controls regulatory T cell functions - PubMed (original) (raw)
. 2015 Feb;125(2):859-69.
doi: 10.1172/JCI79014. Epub 2015 Jan 20.
Bill X Wu, Alessandra Metelli, Jessica E Thaxton, Feng Hong, Saleh Rachidi, Ephraim Ansa-Addo, Shaoli Sun, Chenthamarakshan Vasu, Yi Yang, Bei Liu, Zihai Li
- PMID: 25607841
- PMCID: PMC4319419
- DOI: 10.1172/JCI79014
GP96 is a GARP chaperone and controls regulatory T cell functions
Yongliang Zhang et al. J Clin Invest. 2015 Feb.
Abstract
Molecular chaperones control a multitude of cellular functions via folding chaperone-specific client proteins. CD4+FOXP3+ Tregs play key roles in maintaining peripheral tolerance, which is subject to regulation by multiple molecular switches, including mTOR and hypoxia-inducible factor. It is not clear whether GP96 (also known as GRP94), which is a master TLR and integrin chaperone, controls Treg function. Using murine genetic models, we demonstrated that GP96 is required for Treg maintenance and function, as loss of GP96 resulted in instability of the Treg lineage and impairment of suppressive functions in vivo. In the absence of GP96, Tregs were unable to maintain FOXP3 expression levels, resulting in systemic accumulation of pathogenic IFN-γ-producing and IL-17-producing T cells. We determined that GP96 serves as an essential chaperone for the cell-surface protein glycoprotein A repetitions predominant (GARP), which is a docking receptor for latent membrane-associated TGF-β (mLTGF-β). The loss of both GARP and integrins on GP96-deficient Tregs prevented expression of mLTGF-β and resulted in inefficient production of active TGF-β. Our work demonstrates that GP96 regulates multiple facets of Treg biology, thereby placing Treg stability and immunosuppressive functions strategically under the control of a major stress chaperone.
Figures
Figure 8. GP96 controls mTGF-β bioactivity.
(A) MACS-purified CD4+CD25+ Tregs were stimulated with plate-bound antibody against CD3 (1 μg/ml) and CD28 (0.5 μg/ml) for indicated times. TGF-β levels in the culture supernatant were quantitated by ELISA. Data represent 3 independent experiments. (B) CD4+CD25+ Tregs were stimulated with TGF-β1 (1 ng/ml) for 45 minutes or antibodies against CD3 and CD28 for 24 hours. Cells were then fixed and stained intracellularly for p-Smad2/3. Numbers indicate percentages of indicated cells in the entire population. Data represent 3 independent experiments. (C) CD4+CD25+ Tregs were activated with plate-bound anti-CD3 antibody (2 μg/ml) for 2 to 3 days, followed by irradiation (2000 cGy) and coculturing with CD4+CD25– naive T cells for 3 days, with or without RGD peptide. The level of IL-17A in the culture supernatant was determined by ELISA. Data represent 4 independent experiments. (D and E) 1 × 106 CD4+CD25– naive T cells from 3-week-old WT NOD or Hsp90b1fl/flCD4-Cre mice were transferred into NOD Rag–/– mice. Four weeks later, induced Tregs (CD4+CD25+FOXP3+) were examined by flow cytometry (D) and quantified (E) (each dot represents 1 individual mouse). Data represent 3 independent experiments. (F) Irradiated NOD Rag–/– recipient mice were transplanted with KO BM. Seven days later, mice were injected i.p. with recombinant TGF-β2 (0.2 μg/100 μl) daily for 5 days, followed by once every 3 days. Survival of the mice was monitored. Data represent 2 independent experiments. Statistical analyses were performed with 2-tailed Student’s t test for (A, C, and E) and log-rank test (F).
Figure 7. GP96 is a critical chaperone for cell-surface expression of GARP and mLTGF-β.
(A) Flow cytometry analysis of GARP and mLTGF-β expression on CD4+FOXP3+ Tregs from the thymus and spleen either immediately after isolation or after anti-CD3 and anti-CD28 antibody treatment for 24 hours. (B) Flow cytometry analysis of GARP and mLTGF-β expression on CD41+ platelets. Two experiments were performed with similar findings. (C) Surface expression of GARP or IC GARP (solid line, open histogram) was analyzed in WT and GP96-deficient cells transduced with GARP-FLAG. Gray histograms represent isotype controls. Data from A and C represent 4 independent experiments. (D) Immunoblot of GP96 and GARP-FLAG following immunoprecipitation with GP96 antibody (left) or FLAG antibody (right) from GARP-FLAG–overexpressed cell lysates. Data represent 4 independent experiments. (E) The sensitivity of GARP to _N_-glycase Endo H and PNGase F in WT and GP96 mutant cells. Data are representative of 2 independent experiments. (F) GP96 mutant cells were transduced with full-length GP96 or CBD-deleted GP96 (ΔCBD), followed by examination of cell-surface and IC GARP. Data represent 3 independent experiments. (G) Half-life analysis of GARP-FLAG by immunoblot in WT and GP96-deficient cells following cycloheximide (CHX) treatment. Graph represents densitometric value of the full-length GARP, with time 0 set at 100%. Data represent 2 independent experiments.
Figure 6. GP96-null Tregs lose FOXP3 expression and convert to IFN-γ–producing ex-FOXP3 T cells.
(A) FOXP3 and GP96 expression in Tregs from WT and KO mice was determined by Western blot. Data represent 2 independent experiments. (B) Mean fluorescence intensity (MFI) of FOXP3 stain of Tregs from Het and KO mice. Data are represented as mean ± SEM. (C) Surface expression of β2 integrin (CD18) on Tregs and Teff cells from WT and KO mice was determined by flow cytometry. Numbers represent percentages of CD18– cells in the gated populations. (D) CD4+CD25+ Tregs from WT and KO mice were stimulated with plate-bound antibodies against CD3 and CD28 plus IL-12 for 3 days, followed by IC staining for FOXP3 and IFN-γ. Numbers indicate percentages of indicated cells in the entire population. Two independent experiments were performed with similar findings. (E and F) FACS-sorted CD4+FOXP3GFP+ Tregs (2 × 105) from 3-week-old WT or KO mice were transferred to NOD Rag–/– mice. IC FOXP3 levels in CD4 T cells from the peripheral blood were then analyzed 4 and 6 weeks after the adoptive transfer. The percentage of FOXP3– cells within CD4+ cells (mean ± SEM) was plotted in F. (G and H) IFN-γ levels (percentages) in CD4 T cells from splenocytes of the mice in E and F above were analyzed. Data in C–F represent 4 independent experiments. Two-tailed Student’s t test was used for statistical analysis between groups.
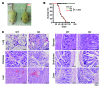
Figure 5. Failure of GP96 KO Tregs to suppress T cell–mediated autoimmune diseases.
(A) 6 × 106 CD25+ T cell–depleted splenocytes from diabetogenic NOD mice were adoptively transferred into NOD Rag–/– mice, along with purified WT or KO Tregs. After transfer, blood glucose was monitored kinetically. (B and C) Analysis of CD4+CD44hiCD62Llo cells in the pancreatic draining LNs of the recipient mice. Two-tailed Student’s t test was used for statistic analysis. One of the 3 representative experiments is shown. (D) H&E staining of pancreatic sections from the recipient mice 2 weeks after the adoptive transfer. (E–H) Suppression of colitis. (E) CD4+CD25–CD45Rbhi T cells isolated from pooled spleen and LNs of NOD mice were transferred i.v. into NOD Rag–/– mice along with either WT or KO Tregs. After transfer, the body weight of the recipient mice was examined weekly. Data represent 2 independent experiments. (F) H&E staining of colon section from the mice 6 weeks after T cell transfer. (G and H) Rescue of autoimmune colitis by WT BM cells. BM cells from WT or KO mice or mixture of the 2 (WT/KO = 1:1) were transfused into lethally irradiated recipients (NOD Rag–/– mice) followed by weekly examination. (G) Body weight. (H) Survival. Data represent results from 3 independent experiments. In experiments depicted in E–H, 2-way ANOVA was used for determining statistical significance on the body weight change; a log-rank (Mantel-Cox) test was used for statistical analysis of mouse survival.
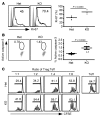
Figure 4. Increased turnover of Tregs and their compromised in vitro suppressive function in the absence of GP96.
(A) IC Ki-67 stain of CD4+FOXP3GFP+ Tregs and quantification of the ratio of Ki-67–positive cells in total Tregs. Data are representative of 2 independent experiments. Two-tailed Student’s t test was used for comparisons of different experimental groups. (B) Treg apoptosis was assayed by active caspase-3 staining. Numbers represent percentages of active caspase-3+ cells among total Tregs. Data are representative of 2 independent experiments. Two-tailed Student’s t test was used for comparisons between Het and KO mice. (C) Suppression of proliferation of CFSE-labeled CD4+CD25– T cells by splenic CD4+CD25+ T cells from KO mice and control littermates. Percentages of CFSElo in the population of all CD4+ cells are indicated. Five individual experiments were performed with similar findings.
Figure 3. Enumeration and phenotypic characterization of GP96-null Tregs.
(A) Enumeration of CD4+FOXP3+ cells in the thymus, spleen, and LN from 4- to 6-week-old mice by flow cytometry. Numbers indicate percentages of gated FOXP3+ cells of all CD4+ cells. n = 8. (B) IC stain of GP96 from FOXP3+ Tregs from the LNs of Het and KO mice. Gray histograms, isotype; open histograms, anti-GP96 antibody. (C) Flow cytometry analysis of cell-surface marker of Tregs from the LNs of KO mice and WT littermates. Shown are representative data from more than 3 independent experiments. (D) Expression of FOXP3 and CD25 in splenic CD4+FOXP3+ Tregs from the indicated mice. Shown are representative data from more than 3 independent experiments.
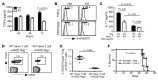
Figure 2. Treg-specific deletion of GP96 in mice triggers systemic cytokine storm and uncontrolled activation of Teff cells.
(A) Serum inflammatory cytokine levels (pg/ml) in WT (n = 2), NOD Het (n = 6), and NOD Foxp3 KO mice (n = 9–10). Data are shown as mean ± SEM. Two-tailed Student’s t test was used for comparisons between Het and KO mice. (B) Flow cytometry analysis of CD44 and CD62L expression of CD4+ T cells in 6-week-old KO mice and Het littermates. Numbers indicate percentages of gated cells of all CD4+ cells. (C) Flow cytometry analysis of IC IFN-γ, IL-4, IL-17, and IL-6 expression by CD4+ T cells from KO mice and Het littermates. Numbers indicate percentages of cells in each quadrant. Representative results from multiple mice are shown.
Figure 1. Foxp3-Cre–mediated Hsp90b1 deletion in mice causes a fatal inflammatory disease.
(A) Rapid loss of body weight of KO mice (right) compared with WT littermates (left). (B) Survival rate of WT (n = 7), Het (n = 10), and KO (n = 18) mice. Mouse survival data was analyzed by a log-rank (Mantel-Cox) test. (C) H&E staining of sections of indicated organs from 7-week-old KO mice and WT littermates. Representative results from multiple mice (n > 3) are shown.
Similar articles
- Garp as a therapeutic target for modulation of T regulatory cell function.
Shevach EM. Shevach EM. Expert Opin Ther Targets. 2017 Feb;21(2):191-200. doi: 10.1080/14728222.2017.1275568. Epub 2016 Dec 29. Expert Opin Ther Targets. 2017. PMID: 28001437 Review. - GARP-TGF-β complexes negatively regulate regulatory T cell development and maintenance of peripheral CD4+ T cells in vivo.
Zhou AX, Kozhaya L, Fujii H, Unutmaz D. Zhou AX, et al. J Immunol. 2013 May 15;190(10):5057-64. doi: 10.4049/jimmunol.1300065. Epub 2013 Apr 10. J Immunol. 2013. PMID: 23576681 Free PMC article. - Spatial and functional targeting of intratumoral Tregs reverses CD8+ T cell exhaustion and promotes cancer immunotherapy.
Zhou L, Velegraki M, Wang Y, Mandula JK, Chang Y, Liu W, Song NJ, Kwon H, Xiao T, Bolyard C, Hong F, Xin G, Ma Q, Rubinstein MP, Wen H, Li Z. Zhou L, et al. J Clin Invest. 2024 May 23;134(14):e180080. doi: 10.1172/JCI180080. J Clin Invest. 2024. PMID: 38787791 Free PMC article. - The GARP/Latent TGF-β1 complex on Treg cells modulates the induction of peripherally derived Treg cells during oral tolerance.
Edwards JP, Hand TW, Morais da Fonseca D, Glass DD, Belkaid Y, Shevach EM. Edwards JP, et al. Eur J Immunol. 2016 Jun;46(6):1480-9. doi: 10.1002/eji.201546204. Epub 2016 Apr 23. Eur J Immunol. 2016. PMID: 27062243 Free PMC article. - Immunoregulatory functions and the therapeutic implications of GARP-TGF-β in inflammation and cancer.
Metelli A, Salem M, Wallace CH, Wu BX, Li A, Li X, Li Z. Metelli A, et al. J Hematol Oncol. 2018 Feb 20;11(1):24. doi: 10.1186/s13045-018-0570-z. J Hematol Oncol. 2018. PMID: 29458436 Free PMC article. Review.
Cited by
- Induction of Foxp3 and activation of Tregs by HSP gp96 for treatment of autoimmune diseases.
Xu Y, Liu E, Xie X, Wang J, Zheng H, Ju Y, Chen L, Li C, Zhou X, Li Z, Li X, Meng S. Xu Y, et al. iScience. 2021 Nov 17;24(12):103445. doi: 10.1016/j.isci.2021.103445. eCollection 2021 Dec 17. iScience. 2021. PMID: 34877502 Free PMC article. - Serum Gp96 is a chaperone of complement-C3 during graft-versus-host disease.
Seignez A, Joly AL, Chaumonnot K, Hazoumé A, Sanka M, Marcion G, Boudesco C, Hammann A, Seigneuric R, Jégo G, Ducoroy P, Delarue P, Senet P, Castilla-Llorente C, Solary E, Durey MA, Rubio MT, Hermine O, Kohli E, Garrido C. Seignez A, et al. JCI Insight. 2017 Mar 23;2(6):e90531. doi: 10.1172/jci.insight.90531. JCI Insight. 2017. PMID: 28352659 Free PMC article. - The evolving paradigm of cell-nonautonomous UPR-based regulation of immunity by cancer cells.
Zanetti M, Rodvold JJ, Mahadevan NR. Zanetti M, et al. Oncogene. 2016 Jan 21;35(3):269-78. doi: 10.1038/onc.2015.108. Epub 2015 Apr 20. Oncogene. 2016. PMID: 25893303 Review. - RNA binding protein PCBP1 is an intracellular immune checkpoint for shaping T cell responses in cancer immunity.
Ansa-Addo EA, Huang HC, Riesenberg B, Iamsawat S, Borucki D, Nelson MH, Nam JH, Chung D, Paulos CM, Liu B, Yu XZ, Philpott C, Howe PH, Li Z. Ansa-Addo EA, et al. Sci Adv. 2020 May 29;6(22):eaaz3865. doi: 10.1126/sciadv.aaz3865. eCollection 2020 May. Sci Adv. 2020. PMID: 32523987 Free PMC article. - Modulation of Endoplasmic Reticulum Stress Controls CD4+ T-cell Activation and Antitumor Function.
Thaxton JE, Wallace C, Riesenberg B, Zhang Y, Paulos CM, Beeson CC, Liu B, Li Z. Thaxton JE, et al. Cancer Immunol Res. 2017 Aug;5(8):666-675. doi: 10.1158/2326-6066.CIR-17-0081. Epub 2017 Jun 22. Cancer Immunol Res. 2017. PMID: 28642246 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- AI070604/AI/NIAID NIH HHS/United States
- AI077283/AI/NIAID NIH HHS/United States
- P30 CA138313/CA/NCI NIH HHS/United States
- P01 CA186866/CA/NCI NIH HHS/United States
- R01 AI077283/AI/NIAID NIH HHS/United States
- P30CA138313/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous