Perlecan is recruited by dystroglycan to nodes of Ranvier and binds the clustering molecule gliomedin - PubMed (original) (raw)
. 2015 Feb 2;208(3):313-29.
doi: 10.1083/jcb.201403111.
Marilena Palmisano 2, Yael Eshed-Eisenbach 3, Desirée Zambroni 1, Ernesto Pavoni 1, Cinzia Ferri 1, Stefania Saccucci 1, Sophie Nicole 4, Raija Soininen 5, Karen K McKee 6, Peter D Yurchenco 6, Elior Peles 3, Lawrence Wrabetz 2, M Laura Feltri 7
Affiliations
- PMID: 25646087
- PMCID: PMC4315246
- DOI: 10.1083/jcb.201403111
Perlecan is recruited by dystroglycan to nodes of Ranvier and binds the clustering molecule gliomedin
Cristina Colombelli et al. J Cell Biol. 2015.
Abstract
Fast neural conduction requires accumulation of Na(+) channels at nodes of Ranvier. Dedicated adhesion molecules on myelinating cells and axons govern node organization. Among those, specific laminins and dystroglycan complexes contribute to Na(+) channel clustering at peripheral nodes by unknown mechanisms. We show that in addition to facing the basal lamina, dystroglycan is found near the nodal matrix around axons, binds matrix components, and participates in initial events of nodogenesis. We identify the dystroglycan-ligand perlecan as a novel nodal component and show that dystroglycan is required for the selective accumulation of perlecan at nodes. Perlecan binds the clustering molecule gliomedin and enhances clustering of node of Ranvier components. These data show that proteoglycans have specific roles in peripheral nodes and indicate that peripheral and central axons use similar strategies but different molecules to form nodes of Ranvier. Further, our data indicate that dystroglycan binds free matrix that is not organized in a basal lamina.
© 2015 Colombelli et al.
Figures
Figure 1.
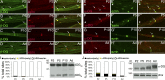
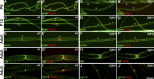
α- and β-DG are early nodal markers. Teased fibers from rat sciatic nerves at P2, P5, P10, and adult (Ad). (A–H) Staining for DG (A–D: green β-DG; E–H: red α-DG) and ezrin (A′–H′) and merged confocal images (A″–H″) show that the majority of early nodes stain for DG. Arrows indicate double positive nodes and asterisks indicate DG-negative nodes. Bar, 35 µm. (I and J) Fraction of nodes positive for the indicated markers at different times. Counts are from two or three experiments from six rats per time point; n = 69 (P2), 68 (P5), 45 (P10), and 20 (Ad) nodes in I and n = 77 (P2), 97 (P5), 24 (P10), and 20 (Ad) nodes in J. Western blot for β-DG (I) or α-DG (J) on developing rat sciatic nerves.
Figure 2.
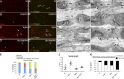
DG is required for proper Na+ channel clustering at nascent nodes and heminodes. (A) Na+ channel clustering is not delayed in DG-deficient nerves. Number of paranodes (Caspr staining) containing (shaded bars) or devoid (open bars) of Na+ channels in nerves. Total sites analyzed for wild type were P2, 89; P5, 362; and P10, 1,107; and for dgko, P2, 74; P5, 336; and P10, 786. Results are reported as mean ± SEM of three mice/genotype. (B and C) Na+ channel clusters form abnormally without DG. Sciatic nerve fibers from P4 and P7 mice stained for pan-Na+ channels. (B) Mutant clusters are smaller and irregular. (C) More clusters are abnormal in DG mutants (P4, n = 83, 79%; P7, n = 41, 69.5% in mutant; P4, n = 27, 23%; P7, n = 15, 23.8% in wild type). P < 0.001 mutant versus wild type by χ2 test; n = 224 (P4) and n = 122 (P7). (D) Abnormal or absent Na+ channel clusters at heminodes are more frequent in dgko (n = 186; 40%) than control (n = 49; 14%). P < 0.001 by χ2 test; n = 823 nodes. (E) Teased nerve fibers from P6 nerves labeled for Na+ channels (red), Caspr (green), and neurofilament (NF; blue). Arrowheads point to the position of developing heminodes/nodes. (F) Staining with S100 (green) shows that SC cytoplasm covers the space between heminodes (arrows). Bars: (B) 4.5 µm; (E and F) 17.5 µm.
Figure 3.
Nodal abnormalities in DGko/Caspr null mutants. (A–D) Teased fibers from P28 sciatic nerves. Staining for Na+ channels (red; A–D) and Caspr (green; A′–D′, merged images). Bar, 17.5 µm. (E) Frequency of normal (asterisk) and abnormal (arrow) clusters in P28 mice nerves (79% in dgko//_Caspr_−/−, n = 102; 69% in dgko, n = 72; 45% in _Caspr_−/−, n = 49; 13% in wild type, n = 16). The difference between dgko//_Caspr_−/− and dgko is not statistically significant by Kruskal-Wallis test. (F–I) EM of nodes in P28 sciatic nerves of wild-type (F), single (G and H), and double mutants (I). SC microvilli (MV) and paranodal loops (PNL) are marked. As reported, paranodal loops detach from the axolemma in _Caspr_−/− and dgko//_Caspr_−/− mice (arrows). SC microvilli of dgko//_Caspr_−/− are disorganized and blunt, and occasionally penetrate between the paranodal loops and the axolemma (arrowheads). An elongated axonal protrusion full of mitochondria is shown (I, asterisk). Bar, 1 µm. (J) Mean nodal length in P28 wild-type, _Caspr_−/−, dgko, and dgko/_Caspr_−/− sciatic nerves by EM (n = 61, 60, 72, and 79, respectively; three mice per genotype). No statistical difference was observed by Student’s t test. (K) The number of nodal axonal spines at P28 is higher in double mutants (9% in wild type, n = 64; 39% in _Caspr_−/−, n = 70; 41% in dgko, n = 80; 82% in dgko//_Caspr_−/−, n = 82). P < 0.001 by χ2 test.
Figure 4.
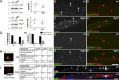
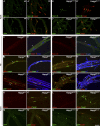
α- and β-DG localize in the nodal gap. IEM on sciatic nerve of wild-type (A and B) or dgko (C and D) adult mouse using antibodies against α-DG (A and C) and β-DG (B and D) shows gold particles decorating SC microvilli both near the basal lamina (arrows in A′ and B′, magnified at right), near the axon, and in the nodal gap (arrowheads in A′ and B′, magnified at right) only in wild-type nerves. Occasional gold grains are randomly distributed in the knockout nerves (C′ and D′, arrows). (E and F) Number of gold grains in each half/node. *, P < 0.02; ***, P < 0.0002; ****, P < 0.0001 by Student’s t test. Error bars represent SEM. Bars: (A–D) 1 µm; (A′–D′) 0.5 µm.
Figure 5.
Increased internodal and decreased nodal ERM in the absence of DG. (A) Western blot of sciatic nerve lysates from wild-type and DG-deficient mice, with antibodies against ERM or phophorylated ERM (ERM-P), normalized to NF155. (right) Ratio of ERM/NF155 and ERM-P/NF155 on three mice per genotype; bars represent SEM. Differences are not significant by Student’s t test. (B–E) Teased fibers from wild-type and dgko adult sciatic nerve immunostained for ezrin (B and C, green), ERM-P (D and E, green), and Caspr (red). (B′–E′) merged images. Note increased ezrin-positive puncta (C, arrows) and lower levels of ERM-P at some dgko nodes (E–E′, asterisks). Double arrowheads indicate nodes with normal amount of ERMs. (G) The percentage of nodes and heminodes (flanked by Caspr) that contain ERM-P were decreased in P6 DG-deficient mice. n = 3 mice/genotype; P < 0.0001 for nodes and P < 0.01 for heminodes by Fisher’s test. (H and I) Teased fibers were stained for Nav 1.6 and ERM-P, and the number of normal and abnormal clusters were correlated with ERM-P staining (examples of a normal Nav cluster, ERM positive, and of an abnormal Nav clusters, ERMP negative, are shown) The absence of p-ERM at nodes correlated with abnormal Na+ channel clusters at P6. P < 0.0001 at P6 and P = 0.09 at P90 by χ2 test. (F and F′) ERM (green) puncta (arrows in F and asterisks in F′) in Cajal bands (containing phalloidin-labeled f-actin, red) colocalize with microtubules (stained with tubulin, blue). Bars: (B–E′) 17.5 µm; (F, H, and I) 5 µm.
Figure 6.
α-DG fragments can be secreted and bind SCs and the ECM via the mucin-like domain. (A) Native α-DG-N is cleaved by furin proteases in rat SCs. SCs were cultured with (+) or without (−) furin inhibitor I (CMK). Western blot of SC lysate and culture medium with an anti–α-DG (IIH6) or an anti–α-DG-N antibody shows that α-DG-N is secreted in the medium and a 120-kD α-DG band is in cells. CMK inhibits α-DG cleavage, as shown by the higher molecular mass (160 kD) of α-DG in SCs and absence of α-DG-N in the medium. Calnexin (clnx) is a loading control. (B) Schematic representation of α-DG, showing its domain composition (N-terminal α-DG-N, mucin-like, transmembrane [TM]; sites of furin and MMP cleavage) and of DGFc proteins DGFc5 (whole α-DG-Fc), DGFc6 (deletion of α-DG-N-Fc), and DGFc2 (α-DG-N). (C) Binding of DG-Fc fusion proteins to rat SCs revealed with anti-Fc (red) and anti-S100 (green, SC marker) antibodies. Nuclei are labeled with DAPI (blue). DGFc5 and DGFc6, but not DGFc2, bind to the SC surface and the associated matrix. Gldn-ECDFc binds mainly to the ECM. (D) Binding of Fc fusion proteins to DRG neurons. Only Gldn-OlfFc binds neurons. (E) Binding of DG-Fc fusion proteins to SC-DRG co-cultures after 10 d in myelinating media. Fc binding is in red and neurofilaments are in green. DGFc5 and DGFc6 bind to the ECM and DGFc2 occasionally binds to SCs aligned with axons (arrows). Bar, 17.5 µm.
Figure 7.
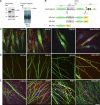
Perlecan is at nodes of Ranvier and is selectively lost in the absence of DG. Sciatic nerve fibers from wild-type and dgko mice. Staining for agrin (agr), perlecan (pcan), or syndecan-3 (syn-3; green; A–J) and Caspr (merged confocal images in A′–J′). Perlecan is enriched at wild type developing (A and C) and adult (E) nodes (arrows). In the absence of DG, perlecan is lost at nodes, but not in the basal lamina (B, D, and F, asterisks). 68% and 87% Caspr-positive paranodes flank perlecan-positive nodes at P6 and P10 in wild-type nerves. Only 4% and 6% of nodes are perlecan positive in DG-deficient age-matched animals. (G–J′) Agrin (G and H) and syndecan-3 (I and J) are at nodes, but they are retained in the absence of DG (G′–J′). Bars, 17.5 µm.
Figure 8.
Na+ channel clusters are retained at nodes or heminodes of two different perlecan mutants. (A–D) Sciatic nerve fibers from adult wild-type (A and C) and _Hspg2_Δ3/Δ3 (B and D) mice. Immunostaining for Na+ channel 1.6 (A and B, green), gliomedin (C and D, green), and Caspr (A′–D′, red) shows no alterations in nodal clusters. (E–N) Sciatic nerve teased fibers from P6 (E–J) or adult (K–N) _Hspg2_KI/+ (controls) and hypomorphic _Hspg2_KI/KO mice. Staining for Na+ channel 1.6 (E and F, red) and perlecan (E′ and F′, green). Perlecan is enriched at nodes of controls (E′, asterisks), but its expression is almost absent in _Hspg2_KI/KO nerves and nodes (F′, asterisk). Immunostaining for pan-Na+ channel (G, H, K, and L, red) and Caspr (G′, H′, K′, and L′, green) or gliomedin (I, J, M, and N, green) and Caspr (I′ and J′, red) show normal accumulation of gliomedin and formation of clusters at heminodes (I and J, arrows) and nodes (G and H, arrows) in perlecan hypomorphs. NF, neurofilament (blue). Bar, 17.5 µm.
Figure 9.
Perlecan binds the collagen domain of gliomedin and increases gliomedin binding to axons and formation of Na+ channel clusters. (A) Comassie blue staining of recombinant perlecan after the first and second step of purification (1 and 2) shows a single band of high molecular mass, consistent with the predicted size of perlecan (arrow). (B) Gliomedin-Fc fusion proteins and Far Western strategy. The whole extracellular domains of NF186, NrCAM, and DG were also used as fusion proteins. (C) Spotting increasing concentration of perlecan shows that only DG and gliomedin, via the collagen domain, bind perlecan. Addition of heparin inhibits the binding. (D) Spotting constant amounts of perlecan (0.5 pM) and overlying increasing amounts of purified Fc-fusion proteins shows that perlecan binding to gliomedin is dose dependent. To confirm equal loading, filters containing the perlecan spot were cut in two and the top half was hybridized with anti-perlecan antibodies. (E) Adult teased fibers from sciatic nerves stained for gliomedin (red) and perlecan (green) show that the two molecules colocalize at nodes (arrows). (F, H, and K) Nodal clustering was induced on DRG neurons by preclustered Fc-gliomedin (gldn) ECD or OLF. Staining of gliomedin clusters (arrows) with anti-Fc antibodies (red) shows that they contain Na+ channels (F, blue), βIV spectrin (H, I, and K, green), and perlecan (F, green). Perlecan colocalized more frequently with Gldn-ECD clusters than Gldn-OLF clusters (G). n = 585 from a single experiment. (H and I) Perlecan increases Gldn ECD-Fc binding to neurons and formation of clusters that contained gldnECD and βIV spectrin (arrows). Neurons are stained with neurofilament (cyan). (J) The number and size of clusters containing gldnECD and βIV spectrin per field of view from six coverslips and two independent experiments were counted; n = 29 fields. Bars represent SEM and statistical significance was evaluated by Student’s t test. (K) Gldn-Fc induced clusters (arrowheads) that co-clustered with βIV spectrin, but not with the transmembrane paranodal protein Caspr. (L) βIV spectrin was diffuse along axons before clustering. Bars: (F, H, I, K, and L) 5 µM; (E) 10 µM.
Figure 10.
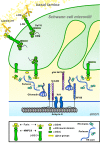
Model for DG and perlecan participation in Na+ channel clustering at nodes. DG aids axonal Na+ channel clustering via different, not mutually exclusive, mechanisms. At the side of basal lamina, DG interaction with laminin 211 regulates the formation of Cajals bands, which may favor ERM transport and remodeling of the microvilli. In the nodal gap, α-DG retains perlecan, which binds gliomedin and favors its binding to axons. It is not known if α-DG at nodes is anchored to the membrane of microvilli or released after cleavage by furin or metalloproteinases. It is possible that two sequential cleavages, by MMPs and furin, release two forms of α-DG with distinct functions in Na+ channel clustering.
Similar articles
- Mechanisms of node of Ranvier assembly.
Rasband MN, Peles E. Rasband MN, et al. Nat Rev Neurosci. 2021 Jan;22(1):7-20. doi: 10.1038/s41583-020-00406-8. Epub 2020 Nov 25. Nat Rev Neurosci. 2021. PMID: 33239761 Review. - Both laminin and Schwann cell dystroglycan are necessary for proper clustering of sodium channels at nodes of Ranvier.
Occhi S, Zambroni D, Del Carro U, Amadio S, Sirkowski EE, Scherer SS, Campbell KP, Moore SA, Chen ZL, Strickland S, Di Muzio A, Uncini A, Wrabetz L, Feltri ML. Occhi S, et al. J Neurosci. 2005 Oct 12;25(41):9418-27. doi: 10.1523/JNEUROSCI.2068-05.2005. J Neurosci. 2005. PMID: 16221851 Free PMC article. - Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier.
Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR Jr, Peles E. Eshed Y, et al. Neuron. 2005 Jul 21;47(2):215-29. doi: 10.1016/j.neuron.2005.06.026. Neuron. 2005. PMID: 16039564 - Long-term maintenance of Na+ channels at nodes of Ranvier depends on glial contact mediated by gliomedin and NrCAM.
Amor V, Feinberg K, Eshed-Eisenbach Y, Vainshtein A, Frechter S, Grumet M, Rosenbluth J, Peles E. Amor V, et al. J Neurosci. 2014 Apr 9;34(15):5089-98. doi: 10.1523/JNEUROSCI.4752-13.2014. J Neurosci. 2014. PMID: 24719088 Free PMC article. - Molecular constituents of the node of Ranvier.
Kazarinova-Noyes K, Shrager P. Kazarinova-Noyes K, et al. Mol Neurobiol. 2002 Oct-Dec;26(2-3):167-82. doi: 10.1385/MN:26:2-3:167. Mol Neurobiol. 2002. PMID: 12428754 Review.
Cited by
- A dual role for Integrin α6β4 in modulating hereditary neuropathy with liability to pressure palsies.
Poitelon Y, Matafora V, Silvestri N, Zambroni D, McGarry C, Serghany N, Rush T, Vizzuso D, Court FA, Bachi A, Wrabetz L, Feltri ML. Poitelon Y, et al. J Neurochem. 2018 May;145(3):245-257. doi: 10.1111/jnc.14295. Epub 2018 Feb 13. J Neurochem. 2018. PMID: 29315582 Free PMC article. - Gene Expression Profiling of the Extracellular Matrix Signature in Macrophages of Different Activation Status: Relevance for Skin Wound Healing.
Etich J, Koch M, Wagener R, Zaucke F, Fabri M, Brachvogel B. Etich J, et al. Int J Mol Sci. 2019 Oct 14;20(20):5086. doi: 10.3390/ijms20205086. Int J Mol Sci. 2019. PMID: 31615030 Free PMC article. - Mechanisms of node of Ranvier assembly.
Rasband MN, Peles E. Rasband MN, et al. Nat Rev Neurosci. 2021 Jan;22(1):7-20. doi: 10.1038/s41583-020-00406-8. Epub 2020 Nov 25. Nat Rev Neurosci. 2021. PMID: 33239761 Review. - Dual transgene amelioration of Lama2-null muscular dystrophy.
McKee KK, Yurchenco PD. McKee KK, et al. Matrix Biol. 2023 Apr;118:1-15. doi: 10.1016/j.matbio.2023.03.001. Epub 2023 Mar 5. Matrix Biol. 2023. PMID: 36878377 Free PMC article. - Heparan Sulfates Support Pyramidal Cell Excitability, Synaptic Plasticity, and Context Discrimination.
Minge D, Senkov O, Kaushik R, Herde MK, Tikhobrazova O, Wulff AB, Mironov A, van Kuppevelt TH, Oosterhof A, Kochlamazashvili G, Dityatev A, Henneberger C. Minge D, et al. Cereb Cortex. 2017 Feb 1;27(2):903-918. doi: 10.1093/cercor/bhx003. Cereb Cortex. 2017. PMID: 28119345 Free PMC article.
References
- Apostolski S., Sadiq S.A., Hays A., Corbo M., Suturkova-Milosevic L., Chaliff P., Stefansson K., LeBaron R.G., Ruoslahti E., Hays A.P., et al. . 1994. Identification of Gal(β1–3)GalNAc bearing glycoproteins at the nodes of Ranvier in peripheral nerve. J. Neurosci. Res. 38:134–141 10.1002/jnr.490380203 - DOI - PubMed
- Bangratz M., Sarrazin N., Devaux J., Zambroni D., Echaniz-Laguna A., René F., Boërio D., Davoine C.S., Fontaine B., Feltri M.L., et al. . 2012. A mouse model of Schwartz-Jampel syndrome reveals myelinating Schwann cell dysfunction with persistent axonal depolarization in vitro and distal peripheral nerve hyperexcitability when perlecan is lacking. Am. J. Pathol. 180:2040–2055 10.1016/j.ajpath.2012.01.035 - DOI - PMC - PubMed
- Bekku Y., Vargová L., Goto Y., Vorísek I., Dmytrenko L., Narasaki M., Ohtsuka A., Fässler R., Ninomiya Y., Syková E., and Oohashi T.. 2010. Bral1: its role in diffusion barrier formation and conduction velocity in the CNS. J. Neurosci. 30:3113–3123 10.1523/JNEUROSCI.5598-09.2010 - DOI - PMC - PubMed
- Bhat M.A., Rios J.C., Lu Y., Garcia-Fresco G.P., Ching W., St Martin M., Li J., Einheber S., Chesler M., Rosenbluth J., et al. . 2001. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 30:369–383 10.1016/S0896-6273(01)00294-X - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DK036425/DK/NIDDK NIH HHS/United States
- NS50220/NS/NINDS NIH HHS/United States
- R01 DK036425/DK/NIDDK NIH HHS/United States
- R01 NS050220/NS/NINDS NIH HHS/United States
- NS055256/NS/NINDS NIH HHS/United States
- DK36425/DK/NIDDK NIH HHS/United States
- R01 NS055256/NS/NINDS NIH HHS/United States
- R01 NS045630/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases