Development and validation of panoptic Meso scale discovery assay to quantify total systemic interleukin-6 - PubMed (original) (raw)
Development and validation of panoptic Meso scale discovery assay to quantify total systemic interleukin-6
Shalini Chaturvedi et al. Br J Clin Pharmacol. 2015 Oct.
Abstract
Aim: Interleukin-6 (IL-6), a multifunctional cytokine, exists in several forms ranging from a low molecular weight (MW 20-30 kDa) non-complexed form to high MW (200-450 kDa), complexes. Accurate baseline IL-6 assessment is pivotal to understand clinical responses to IL-6-targeted treatments. Existing assays measure only the low MW, non-complexed IL-6 form. The present work aimed to develop a validated assay to measure accurately total IL-6 (complexed and non-complexed) in serum or plasma as matrix in a high throughput and easily standardized format for clinical testing.
Methods: Commercial capture and detection antibodies were screened against humanized IL-6 and evaluated in an enzyme-linked immunosorbent assay format. The best antibody combinations were screened to identify an antibody pair that gave minimum background and maximum recovery of IL-6 in the presence of 100% serum matrix. A plate-based total IL-6 assay was developed and transferred to the Meso Scale Discovery (MSD) platform for large scale clinical testing.
Results: The top-performing antibody pair from 36 capture and four detection candidates was validated on the MSD platform. The lower limit of quantification in human serum samples (n = 6) was 9.77 pg l(-1) , recovery ranged from 93.13-113.27%, the overall pooled coefficients of variation were 20.12% (inter-assay) and 8.67% (intra-assay). High MW forms of IL-6, in size fractionated serum samples from myelodysplastic syndrome and rheumatoid arthritis patients, were detected by the assay but not by a commercial kit.
Conclusion: This novel panoptic (sees all forms) IL-6 MSD assay that measures both high and low MW forms may have clinical utility.
Keywords: cancer; interleukin-6; panoptic assay; rheumatoid arthritis.
© 2015 The British Pharmacological Society.
Figures
Figure 1
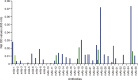
Capture antibodies screened for IL-6 capture in combination with four different detection antibodies. Each candidate antibody was used as a capture antibody to assess 125 pg ml–1 human recombinant IL-6. Detection antibodies from commercially available ELISA kits were used to determine which antibody pair would result in the best detection of IL-6 in human serum. The approach was repeated for human plasma samples, with similar results (data not shown). IL-6, interleukin 6; ELISA, enzyme-linked immunosorbent assay; OD, optical density; mAb, monoclonal antibody; det Ab, detection antibody;  Det Ab-1,
Det Ab-1,  Det Ab-2,
Det Ab-2,  Det Ab-3,
Det Ab-3,  Det Ab-4
Det Ab-4
Figure 2
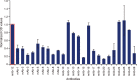
Comparison of capture antibodies in the second round of screening. Capture antibodies that passed the broad first round screening were further evaluated in standard ELISA format. Thermo-Pierce 5IL6 (mAb-12; circled) consistently emerged as a superior capture antibody and was included in all ELISAs to normalize ELISA plate runs. ELISA, enzyme-linked immunosorbent assay; OD, optical density; mAb, monoclonal antibody
Figure 3
Comparison of top-performing detection and capture antibodies. (A) Four detection antibodies from commercial IL-6 kits were screened and compared for their ability to recognize human recombinant IL-6. Thermo-Pierce 5IL6 was used as a capture antibody. Invitrogen detection antibody (505E23C7) was selected as the detection antibody for future screenings. (B) The three top-performing capture monoclonal anti-IL-6 antibodies (Thermo-Pierce 5IL6, Ebiosciences 16YOR5/66 and Epitomics EBI-R14-19) were compared in a single analysis. The detection antibody was Invitrogen ELISA detection antibody 505E23C7. IL-6, interleukin 6; hIL6, human interleukin 6; ELISA, enzyme-linked immunosorbent assay; OD, optical density; det Ab, detection antibody,  R&D det mAb,
R&D det mAb,  Thermo-Pierce det mAb,
Thermo-Pierce det mAb,  PeproTech det mAb,
PeproTech det mAb,  Invitrogen det mAb,
Invitrogen det mAb,  Thermo-Pierce 5IL6,
Thermo-Pierce 5IL6,  Ebiosciences 16YOR5/66,
Ebiosciences 16YOR5/66,  Epitomics EBI-R14-19
Epitomics EBI-R14-19
Figure 4
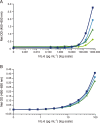
Final antibody pair selection for determining the best pair that gives the maximum spread of values. Capture/detection antibody pairs evaluated shown respectively: (A) CNTO328/Invitrogen 505E23C7, (B) Thermo-Pierce 5IL6/Invitrogen 505E23C7, (C) Epitomics EBI-R14-19/Invitrogen 505E23C7, (D) MSD IL-6 kit assay and (E) MSD IL-6 kit assay with the Humanzyme IL-6 standard. Solid line indicates LLOQ at 9.77 pg ml–1 and dotted line indicates LLOQ at 1.22 pg ml–1. IL-6, interleukin 6; HS, human serum; RA, rheumatoid arthritis; MSD, Meso Scale Discovery; LLOQ, lower limit of quantification
Figure 5
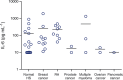
IL-6 concentrations in normal and disease sera samples as measured by panoptic IL-6, MSD assay; IL-6, interleukin 6; MSD, Meso Scale Discovery; HS, human serum; RA, rheumatoid arthritis
Figure 6
Superdex 200 gel filtration and immunologic characterization of IL-6 in serum from patients with RA (n = 7) and patients with MDS (n = 6). (A) IL-6 concentrations of each fraction assayed using commercial MSD assay and (B) IL-6 concentrations of each fraction assayed using the panoptic IL-6 assay. IL-6, interleukin 6; MDS, myelodysplastic syndrome; MSD, Meso Scale Discovery; SEC, size exclusion column,  RA 1,
RA 1,  RA 2,
RA 2,  RA 3,
RA 3,  RA 4,
RA 4,  RA 5,
RA 5,  RA 6,
RA 6,  RA 7,
RA 7,  MDS 1,
MDS 1,  MDS 2,
MDS 2,  MDS 3,
MDS 3,  MDS 4,
MDS 4,  MDS 5,
MDS 5,  MDS 6
MDS 6
Similar articles
- Measurement of IL-21 in human serum and plasma using ultrasensitive MSD S-PLEX® and Quanterix SiMoA methodologies.
Poorbaugh J, Samanta T, Bright SW, Sissons SE, Chang CY, Oberoi P, MacDonald AJ, Martin AP, Cox KL, Benschop RJ. Poorbaugh J, et al. J Immunol Methods. 2019 Mar;466:9-16. doi: 10.1016/j.jim.2018.12.005. Epub 2018 Dec 24. J Immunol Methods. 2019. PMID: 30590020 - Analytical validation of a highly sensitive microparticle-based immunoassay for the quantitation of IL-13 in human serum using the Erenna immunoassay system.
St Ledger K, Agee SJ, Kasaian MT, Forlow SB, Durn BL, Minyard J, Lu QA, Todd J, Vesterqvist O, Burczynski ME. St Ledger K, et al. J Immunol Methods. 2009 Oct 31;350(1-2):161-70. doi: 10.1016/j.jim.2009.08.012. Epub 2009 Sep 2. J Immunol Methods. 2009. PMID: 19732777 - Validation of a multiplex electrochemiluminescent immunoassay platform in human and mouse samples.
Bastarache JA, Koyama T, Wickersham NE, Ware LB. Bastarache JA, et al. J Immunol Methods. 2014 Jun;408:13-23. doi: 10.1016/j.jim.2014.04.006. Epub 2014 Apr 21. J Immunol Methods. 2014. PMID: 24768796 Free PMC article. - Evaluation of heterophilic antibody blocking agents in reducing false positive interference in immunoassays for IL-17AA, IL-17FF, and IL-17AF.
DeForge LE, Loyet KM, Delarosa D, Chinn J, Zamanian F, Chuntharapai A, Lee J, Hass P, Wei N, Townsend MJ, Wang J, Wong WL. DeForge LE, et al. J Immunol Methods. 2010 Oct 31;362(1-2):70-81. doi: 10.1016/j.jim.2010.09.004. Epub 2010 Sep 9. J Immunol Methods. 2010. PMID: 20833179 - Comparative Analysis of Multiple Immunoassays for Cytokine Profiling in Drug Discovery.
Platchek M, Lu Q, Tran H, Xie W. Platchek M, et al. SLAS Discov. 2020 Dec;25(10):1197-1213. doi: 10.1177/2472555220954389. Epub 2020 Sep 13. SLAS Discov. 2020. PMID: 32924773 Review.
Cited by
- Interleukin-6 is a potential therapeutic target in interleukin-6 dependent, estrogen receptor-α-positive breast cancer.
Casneuf T, Axel AE, King P, Alvarez JD, Werbeck JL, Verhulst T, Verstraeten K, Hall BM, Sasser AK. Casneuf T, et al. Breast Cancer (Dove Med Press). 2016 Feb 3;8:13-27. doi: 10.2147/BCTT.S92414. eCollection 2016. Breast Cancer (Dove Med Press). 2016. PMID: 26893580 Free PMC article. - Integrated Network Pharmacology and Metabolomics Analysis of the Therapeutic Effects of Zi Dian Fang on Immune Thrombocytopenic Purpura.
Li Y, Li Y, Lu W, Li H, Wang Y, Luo H, Wu Y, Dong W, Bai G, Zhang Y. Li Y, et al. Front Pharmacol. 2018 Jun 19;9:597. doi: 10.3389/fphar.2018.00597. eCollection 2018. Front Pharmacol. 2018. PMID: 29971001 Free PMC article. - Tumor-derived IL-6 and trans-signaling among tumor, fat, and muscle mediate pancreatic cancer cachexia.
Rupert JE, Narasimhan A, Jengelley DHA, Jiang Y, Liu J, Au E, Silverman LM, Sandusky G, Bonetto A, Cao S, Lu X, O'Connell TM, Liu Y, Koniaris LG, Zimmers TA. Rupert JE, et al. J Exp Med. 2021 Jun 7;218(6):e20190450. doi: 10.1084/jem.20190450. J Exp Med. 2021. PMID: 33851955 Free PMC article. - Interleukin-6 at the Host-Tumor Interface: STAT3 in Biomolecular Condensates in Cancer Cells.
Sehgal PB. Sehgal PB. Cells. 2022 Mar 30;11(7):1164. doi: 10.3390/cells11071164. Cells. 2022. PMID: 35406728 Free PMC article. Review. - Linking physiological parameters to perturbations in the human exposome: Environmental exposures modify blood pressure and lung function via inflammatory cytokine pathway.
Stiegel MA, Pleil JD, Sobus JR, Stevens T, Madden MC. Stiegel MA, et al. J Toxicol Environ Health A. 2017;80(9):485-501. doi: 10.1080/15287394.2017.1330578. Epub 2017 Jul 11. J Toxicol Environ Health A. 2017. PMID: 28696913 Free PMC article.
References
- Hirano T. Interleukin-6 and its relation to inflammation and disease. Clin Immunol Immunopathol. 1992;62:S60–5. - PubMed
- Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer: the role of interleukin 6 (IL-6) BJU Int. 2014;113:986–92. - PubMed
- Salgado R, Junius S, Benoy I, Van DP, Vermeulen P, Van ME, Huget P, Dirix LY. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous