The transcription factors Ik-1 and MZF1 downregulate IGF-IR expression in NPM-ALK⁺ T-cell lymphoma - PubMed (original) (raw)
The transcription factors Ik-1 and MZF1 downregulate IGF-IR expression in NPM-ALK⁺ T-cell lymphoma
Deeksha Vishwamitra et al. Mol Cancer. 2015.
Abstract
Background: The type I insulin-like growth factor receptor (IGF-IR) tyrosine kinase promotes the survival of an aggressive subtype of T-cell lymphoma by interacting with nucleophosmin-anaplastic lymphoma kinase (NPM-ALK) oncogenic protein. NPM-ALK(+) T-cell lymphoma exhibits much higher levels of IGF-IR than normal human T lymphocytes. The mechanisms underlying increased expression of IGF-IR in this lymphoma are not known. We hypothesized that upregulation of IGF-IR could be attributed to previously unrecognized defects that inherently exist in the transcriptional machinery in NPM-ALK(+) T-cell lymphoma.
Methods and results: Screening studies showed substantially lower levels of the transcription factors Ikaros isoform 1 (Ik-1) and myeloid zinc finger 1 (MZF1) in NPM-ALK(+) T-cell lymphoma cell lines and primary tumor tissues from patients than in human T lymphocytes. A luciferase assay supported that Ik-1 and MZF1 suppress IGF-IR gene promoter. Furthermore, ChIP assay showed that these transcription factors bind specific sites located within the IGF-IR gene promoter. Forced expression of Ik-1 or MZF1 in the lymphoma cells decreased IGF-IR mRNA and protein. This decrease was associated with downregulation of pIGF-IR, and the phosphorylation of its interacting proteins IRS-1, AKT, and NPM-ALK. In addition, overexpression of Ik-1 and MZF1 decreased the viability, proliferation, migration, and anchorage-independent colony formation of the lymphoma cells.
Conclusions: Our results provide novel evidence that the aberrant decreases in Ik-1 and MZF1 contribute significantly to the pathogenesis of NPM-ALK(+) T-cell lymphoma through the upregulation of IGF-IR expression. These findings could be exploited to devise new strategies to eradicate this lymphoma.
Figures
Figure 1
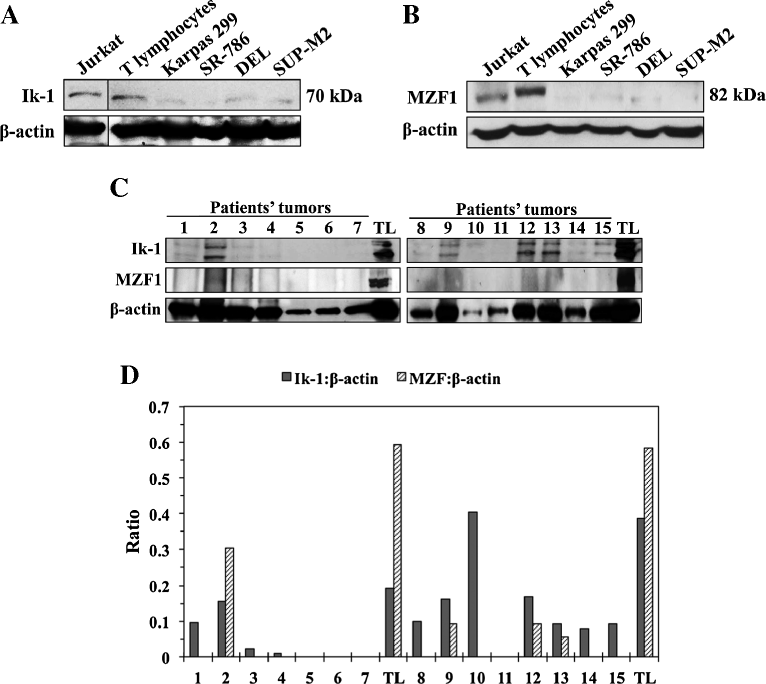
The expression of Ik-1 and MZF1 is decreased in NPM-ALK + T-cell lymphoma and primary tumors from patients. (A) Western blotting shows that Ik-1 levels were markedly lower in 4 NPM-ALK+ T-cell lymphoma cell lines than in T lymphocytes. Jurkat cells were used as a positive control. β-actin showed equal protein loading. Vertical lines have been inserted to indicate repositioned gel lanes. (B) Similarly, MZF1 levels were substantially lower in the lymphoma cell lines than in normal human T-cells. Jurkat cells served as a positive control. β-Actin demonstrated equal levels of loaded proteins. (C) The expression of Ik-1 and MZF1 is shown in 15 ALK+ T-cell lymphoma specimens from patients. Patient’s lysates were divided into 2 groups – 1–7 (left panel) and 8–15 (right panel) – and lysates from 2 different pools of normal human T lymphocytes (TL) were analyzed simultaneously as controls with the corresponding lysates from the patient tumors. Because the quality of the formalin-fixed and paraffin-embedded tissue sections varied significantly, β-actin showed unequal protein levels. (D) Despite apparent differences in protein levels loaded in the different lanes, densitometric analysis of the Ik-1/MZF1:β-actin ratio of the Western blot bands shown in (C) supports that the expression of Ik-1 and MZF1 was markedly reduced in 87% and 100%, respectively, of the ALK+ T-cell lymphoma specimens from patients compared to human TL. The Western blotting assays were performed 3 independent times with consistent results.
Figure 2
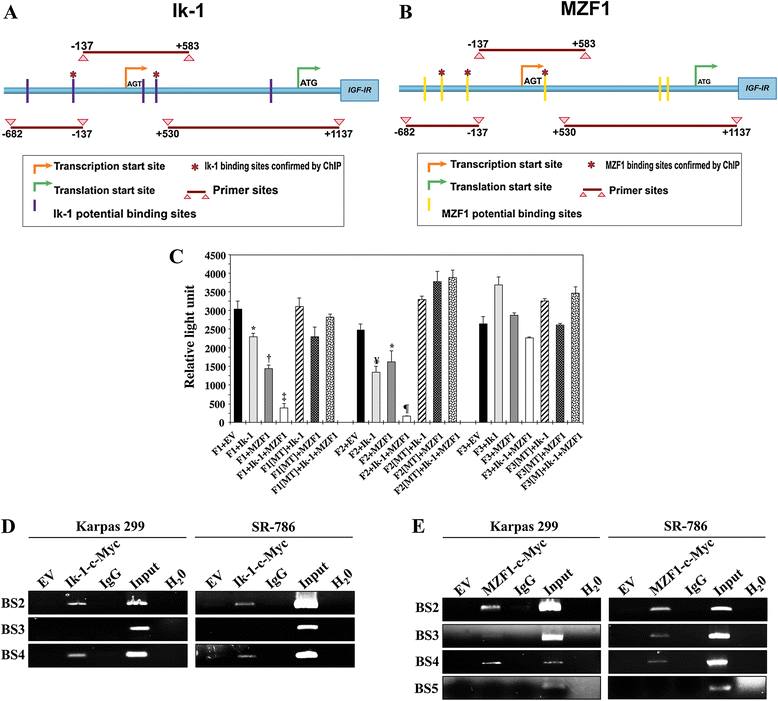
Ik-1 and MZF1 interact with and bind the IGF-IR gene promoter. (A) IGF-IR gene promoter map including the transcription (AGT) and translation (ATG) start sites. The 3 PCR fragments used in the luciferase assay (F1: −682/-137, F2: −137/+583, F3: +530/+1137) and 5 sites that potentially bind with Ik-1 are shown. *: 2 sites confirmed by ChIP assay. (B) In addition to the 3 PCR fragments, 6 sites that potentially bind with MZF1 are depicted. *: 3 sites confirmed by the ChIP. (C) Luciferase assay performed in R− cells demonstrates that transfection of Ik-1 or MZF1 decreased IGF-IR promoter activity with F1 and F2, but not with F3. Cotransfection of Ik-1 and MZF1 induced more pronounced inhibitory effects on F1 and F2 than one transcription factor alone (*: P < 0.05 vs. F1+EV and F2+EV; †: P < 0.01 vs. F1+Ik-1 and P < 0.0001 vs. F1+EV; ‡: P < 0.0001 vs. F1+EV and F1+Ik-1 and P < 0.01 vs. F1+MZF1; ¥: P < 0.001 vs. F2+EV; ¶: P < 0.001 vs. F2+EV and < 0.0001 vs. F2+Ik-1 and F2+MZF1). Ik-1 and MZF1 failed to induce similar effects when F1 and F2 were mutated (MT) at potential binding sites. The results are shown as means ± SE of 3 consistent experiments. (D) ChIP assay shows that Ik-1 binds with binding site 2 (BS2) and BS4, but not BS3, of the promoter. Controls, including EV, IgG, input, and H2O, worked properly. The 2 binding sites are identified by an (*) in (A). (E) ChIP assay confirms that MZF1 binds with the promoter at BS2, BS3, and BS4, and not BS5, which are marked by an (*) in (B). Controls worked properly. Some of the panels shown in (D) and (E) have been slightly enhanced in their entirety to assist with the visualization of the weak bands, which are pertinent to the results.
Figure 3
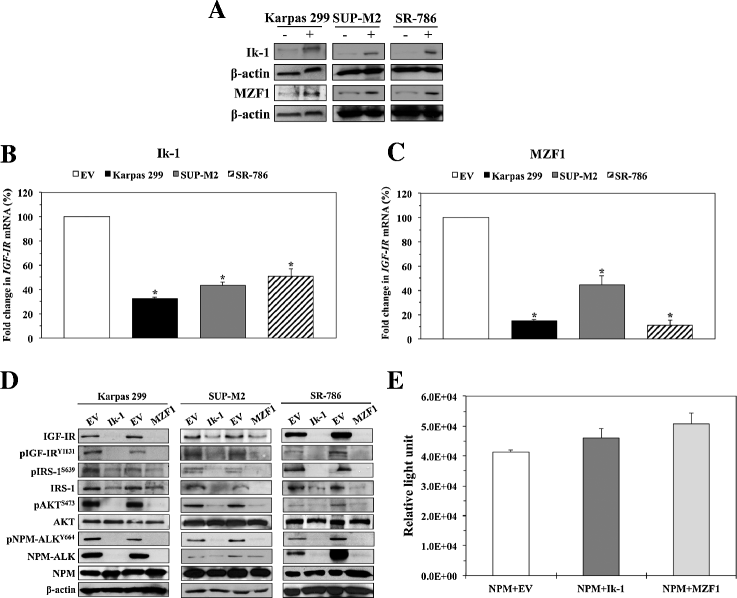
Ik-1 and MZF1 decrease IGF-IR mRNA and protein levels and the phosphorylation of downstream targets. (A) Western blotting demonstrates increased expression levels of Ik-1 and MZF1 proteins at 48 h after transfection into 3 NPM-ALK+ T-cell lymphoma cell lines. β-actin shows equal protein loading (−: transfection of EV; +: transfection of Ik-1 or MZF1). (B) Transfection of Ik-1 remarkably decreased IGF-IR mRNA levels in Karpas 299, SUP-M2, and SR-786 cell lines (*: < 0.0001 compared with EV). (C) Similarly, transfection of MZF1 induced a significant decrease in IGF-IR mRNA levels (*: < 0.0001 compared with EV). The results depicted in (B) and (C) represent the means ± SE of 3 experiments. (D) Western blotting shows that transfection of Ik-1 and MZF1 into Karpas 299, SUP-M2, and SR-786 cell lines induced marked downregulation of IGF-IR protein, which was associated with decreased pIGF-IR levels. Moreover, the decrease in IGF-IR/pIGF-IR expression levels was associated with decreased phosphorylation of important IGF-IR targets including IRS-1, AKT, and NPM-ALK. Whereas basal levels of AKT remained unchanged, notable decrease in IRS-1 protein was observed. The 3 web-based transcription factor search algorithms showed that Ik-1 and MZF1 could potentially bind the IRS-1 gene promoter. In contrast, searching these algorithms did not support potential binding of Ik-1 or MZF1 and the NPM gene promoter, where the expression of NPM-ALK protein is regulated at the transcriptional level. (E) In line with lack of potential binding/interaction between Ik-1 or MZF1 and the NPM gene promoter, a luciferase assay performed in R− cells shows that transfection of Ik-1 and MZF1 does not decrease the NPM promoter activity. The results represent means ± SE of 3 consistent experiments.
Figure 4
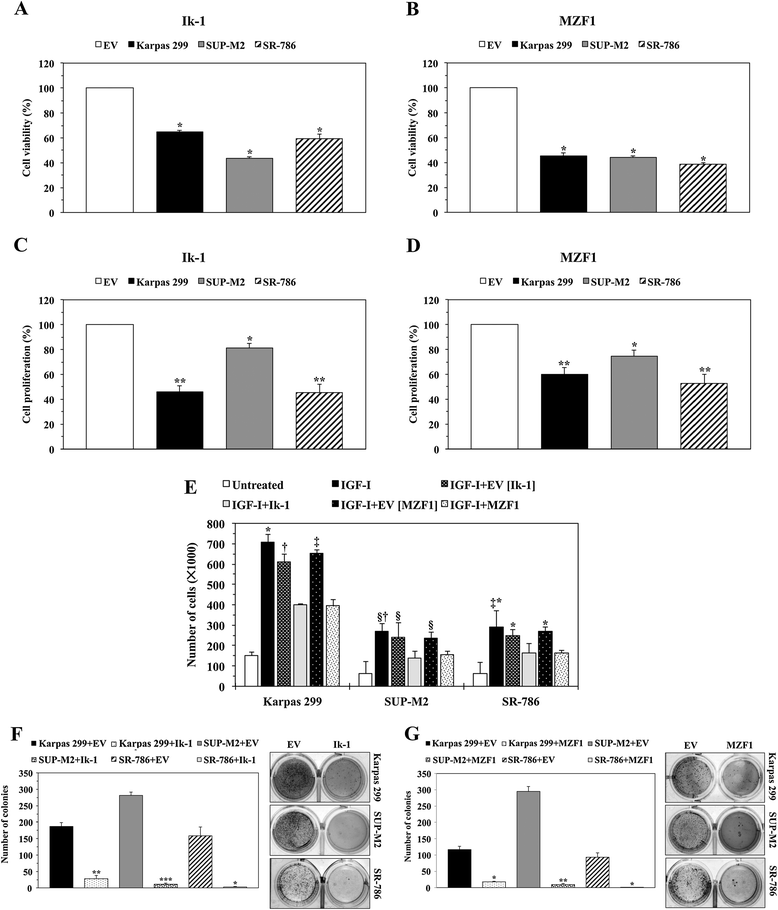
Transfection of Ik-1 and MZF1 decreases the viability, proliferation, migration, and anchorage-independent colony formation of NPM-ALK + T-cell lymphoma cells. (A) Compared with EV, transfection of Ik-1 decreased the viability of the lymphoma cells at 48 h (*: P < 0.0001). (B) In a similar fashion, transfection of MZF1 decreased cellular viability (*: P < 0.0001). (C) Ik-1 decreased cellular proliferation (*: P < 0.05; **: P < 0.01). (D) Transfection of MZF1 also reduced the proliferation of these cells (*: P < 0.05; **: P < 0.01). (E) IGF-I stimulated the migration of the lymphoma cells. Whereas this effect was substantially decreased when cells were treated with IGF-I and transfected with Ik-1 or MZF1; EV failed to induce similar effects (Karpas 299, *: P < 0.0001 in IGF-I vs. IGF-I+Ik-1 and IGF-I+MZF1, †: P < 0.01 in IGF-I+EV [Ik-1] vs. IGF-I+Ik-1, ‡: P < 0.001 in IGF-I+EV [MZF1] vs. IGF-I+MZF1; SUP-M2, §: P < 0.05 in IGF-I vs. IGF-I+Ik-1, IGF-I+EV [Ik-1] vs. IGF-I+Ik-1 and IGF-I+EV [MZF1] vs. IGF-I+MZF1, †: P < 0.01 in IGF-I vs. IGF-I+MZF1; SR-786, *: P < 0.0001 in IGF-I vs. IGF-I+MZF1, IGF-I+EV [Ik-1] vs. IGF-I+Ik-1, and IGF-I+EV [MZF1] vs. IGF-I+MZF1, ‡: P < 0.001 in IGF-I vs. IGF-I+Ik-1). (F) Ik-1 abrogated anchorage-independent colony formation of the lymphoma cells at 7 days after transfection (*: P < 0.05; **: P < 0.01; ***: P < 0.001 compared with EV). The numbers of colonies are shown in the left panel, and examples of the colonies are illustrated in the right panel. (G) Similarly, MZF1 halted the lymphoma cell colony formation (*: P < 0.05; **: P < 0.01 compared with EV). The numbers of colonies are shown in the left panel, and examples of the colonies are depicted in the right panel. Results represent means ± SE of at least 3 consistent experiments.
Figure 5
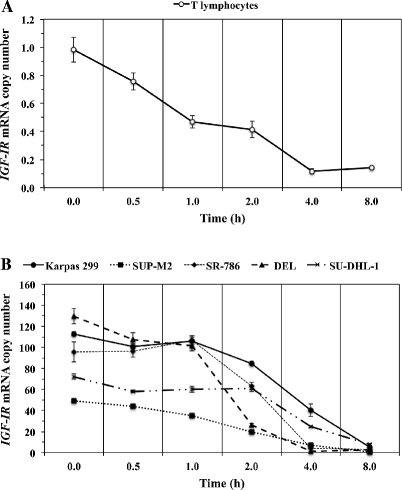
IGF-IR mRNA expressed in NPM-ALK + T-cell lymphoma cells exhibits prolonged decay time compared with IGF-IR mRNA from normal human T lymphocytes. (A) The decay of IGF-IR mRNA in normal human T lymphocytes over 8 h is illustrated. The 50% level (t1/2) of IGF-IR mRNA was detected at 0.8 h. (B) In contrast with T lymphocytes, the NPM-ALK+ T-cell lymphoma cell lines expressed remarkably higher basal levels of IGF-IR mRNA, with DEL and SUP-M2 cells demonstrating the highest and lowest levels, respectively. The t1/2 for IGF-IR mRNA level was achieved after longer periods of time in the lymphoma cells than in normal T lymphocytes (SU-DHL-1: 3.7 h, Karpas 299: 3.4 h, SR-786: 2.6 h, SUP-M2: 1.9 h, DEL: 1.5 h). The mean of the t1/2 IGF-IR mRNA decay time in the lymphoma cells was 2.62 ± 0.4 h (SE). Results shown represent the means ± SE of 3 experiments.
Figure 6
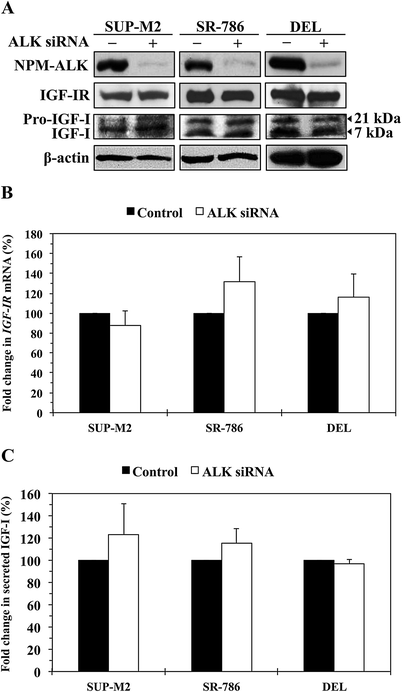
NPM-ALK oncogenic protein does not affect the levels of expression of IGF-IR and IGF-I. (A) Western blotting shows that at 48 h, downregulation of NPM-ALK by ALK siRNA was not associated with decreased expression of IGF-IR, pro-IGF-I or IGF-I proteins in SUP-M2, SR-786, and DEL cell lines. β-Actin shows equal protein loading. Analysis of IGF-IR levels after transfection of the cells with ALK siRNA was performed at extended time points (12, 24, 48, 72, and 96 h), and also in other cell lines including Karpas 299 and SU-DHL-1, with similar results (data not shown). (B) Downregulation of NPM-ALK in the 3 cell lines did not decrease the levels of IGF-IR mRNA. The example shown is at 48 h after transfection of the cells with ALK siRNA. The results are shown as means ± SE of 4 consistent experiments. In addition, analysis of IGF-IR mRNA was performed at other time points and cell lines as described in (A). Changes in IGF-IR mRNA levels were not detected at any time point (data not shown). (C) An ELISA assay showing that specific downregulation of NPM-ALK did not significantly decrease the levels of IGF-I secreted from the NPM-ALK+ T-cell lymphoma cells. The results represent the means ± SE of 3 experiments.
Similar articles
- Molecular and functional characterizations of the association and interactions between nucleophosmin-anaplastic lymphoma kinase and type I insulin-like growth factor receptor.
Shi B, Vishwamitra D, Granda JG, Whitton T, Shi P, Amin HM. Shi B, et al. Neoplasia. 2013 Jun;15(6):669-83. doi: 10.1593/neo.122012. Neoplasia. 2013. PMID: 23730215 Free PMC article. - NPM-ALK up-regulates iNOS expression through a STAT3/microRNA-26a-dependent mechanism.
Zhu H, Vishwamitra D, Curry CV, Manshouri R, Diao L, Khan A, Amin HM. Zhu H, et al. J Pathol. 2013 May;230(1):82-94. doi: 10.1002/path.4171. Epub 2013 Mar 14. J Pathol. 2013. PMID: 23338972 Free PMC article. - TrkA is a binding partner of NPM-ALK that promotes the survival of ALK+ T-cell lymphoma.
Shi W, George SK, George B, Curry CV, Murzabdillaeva A, Alkan S, Amin HM. Shi W, et al. Mol Oncol. 2017 Sep;11(9):1189-1207. doi: 10.1002/1878-0261.12088. Epub 2017 Jun 18. Mol Oncol. 2017. PMID: 28557340 Free PMC article. - Type I insulin-like growth factor receptor signaling in hematological malignancies.
Vishwamitra D, George SK, Shi P, Kaseb AO, Amin HM. Vishwamitra D, et al. Oncotarget. 2017 Jan 3;8(1):1814-1844. doi: 10.18632/oncotarget.12123. Oncotarget. 2017. PMID: 27661006 Free PMC article. Review. - Role and Regulation of Myeloid Zinc Finger Protein 1 in Cancer.
Eguchi T, Prince T, Wegiel B, Calderwood SK. Eguchi T, et al. J Cell Biochem. 2015 Oct;116(10):2146-54. doi: 10.1002/jcb.25203. J Cell Biochem. 2015. PMID: 25903835 Free PMC article. Review.
Cited by
- The Ikaros family of zinc-finger proteins.
Fan Y, Lu D. Fan Y, et al. Acta Pharm Sin B. 2016 Nov;6(6):513-521. doi: 10.1016/j.apsb.2016.06.002. Epub 2016 Jun 24. Acta Pharm Sin B. 2016. PMID: 27818917 Free PMC article. Review. - MZF1 mediates oncogene-induced senescence by promoting the transcription of p16INK4A.
Wu D, Tan H, Su W, Cheng D, Wang G, Wang J, Ma DA, Dong GM, Sun P. Wu D, et al. Oncogene. 2022 Jan;41(3):414-426. doi: 10.1038/s41388-021-02110-y. Epub 2021 Nov 12. Oncogene. 2022. PMID: 34773072 Free PMC article. - Zinc Finger Transcription Factor MZF1-A Specific Regulator of Cancer Invasion.
Brix DM, Bundgaard Clemmensen KK, Kallunki T. Brix DM, et al. Cells. 2020 Jan 16;9(1):223. doi: 10.3390/cells9010223. Cells. 2020. PMID: 31963147 Free PMC article. Review. - MZF1 promotes tumour progression and resistance to anti-PD-L1 antibody treatment in hepatocellular carcinoma.
Kan A, Liu S, He M, Wen D, Deng H, Huang L, Lai Z, Huang Y, Shi M. Kan A, et al. JHEP Rep. 2023 Oct 25;6(1):100939. doi: 10.1016/j.jhepr.2023.100939. eCollection 2024 Jan. JHEP Rep. 2023. PMID: 38074509 Free PMC article. - Dual inhibition of IGF-IR and ALK as an effective strategy to eradicate NPM-ALK+ T-cell lymphoma.
George B, George SK, Shi W, Haque A, Shi P, Eskandari G, Axelson M, Larsson O, Kaseb AO, Amin HM. George B, et al. J Hematol Oncol. 2019 Jul 24;12(1):80. doi: 10.1186/s13045-019-0768-8. J Hematol Oncol. 2019. PMID: 31340850 Free PMC article.
References
- Grønborg M, Wulff BS, Rasmussen JS, Kjeldsen T, Gammeltoft S. Structure-function relationship of the insulin-growth factor-I receptor tyrosine kinase. J Biol Chem. 1993;268:23435–23440. - PubMed
- Bloomfield FH, van Zijl PL, Bauer MK, Phua HH, Harding JE. Effect of pulsatile growth hormone administration to the growth-restricted fetal sheep on somatotrophic axis gene expression in fetal and placental tissues. Am J Endocrinol Metab. 2006;291:E333–E339. doi: 10.1152/ajpendo.00045.2006. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous