Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance - PubMed (original) (raw)
doi: 10.1038/nn.4025. Epub 2015 May 25.
Abhay P Sagare 1, Qingyi Ma 1, Matthew R Halliday 1, Pan Kong 1, Kassandra Kisler 1, Ethan A Winkler 2, Anita Ramanathan 1, Takahisa Kanekiyo 3, Guojun Bu 3, Nelly Chuqui Owens 1, Sanket V Rege 1, Gabriel Si 1, Ashim Ahuja 1, Donghui Zhu 4, Carol A Miller 5, Julie A Schneider 6, Manami Maeda 7, Takahiro Maeda 7, Tohru Sugawara 8, Justin K Ichida 8, Berislav V Zlokovic 1
Affiliations
- PMID: 26005850
- PMCID: PMC4482781
- DOI: 10.1038/nn.4025
Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance
Zhen Zhao et al. Nat Neurosci. 2015 Jul.
Erratum in
- Author Correction: Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance.
Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, Owens NC, Rege SV, Si G, Ahuja A, Zhu D, Miller CA, Schneider JA, Maeda M, Maeda T, Sugawara T, Ichida JK, Zlokovic BV. Zhao Z, et al. Nat Neurosci. 2024 Jan;27(1):208. doi: 10.1038/s41593-023-01509-y. Nat Neurosci. 2024. PMID: 37985802 No abstract available.
Abstract
PICALM is a highly validated genetic risk factor for Alzheimer's disease (AD). We found that reduced expression of PICALM in AD and murine brain endothelium correlated with amyloid-β (Aβ) pathology and cognitive impairment. Moreover, Picalm deficiency diminished Aβ clearance across the murine blood-brain barrier (BBB) and accelerated Aβ pathology in a manner that was reversible by endothelial PICALM re-expression. Using human brain endothelial monolayers, we found that PICALM regulated PICALM/clathrin-dependent internalization of Aβ bound to the low density lipoprotein receptor related protein-1, a key Aβ clearance receptor, and guided Aβ trafficking to Rab5 and Rab11, leading to Aβ endothelial transcytosis and clearance. PICALM levels and Aβ clearance were reduced in AD-derived endothelial monolayers, which was reversible by adenoviral-mediated PICALM transfer. Inducible pluripotent stem cell-derived human endothelial cells carrying the rs3851179 protective allele exhibited higher PICALM levels and enhanced Aβ clearance. Thus, PICALM regulates Aβ BBB transcytosis and clearance, which has implications for Aβ brain homeostasis and clearance therapy.
Figures
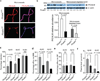
Figure 1. PICALM reductions in brain capillary endothelium in Alzheimer’s disease
a, PICALM and Aβ immunostaining in the prefrontal cortex of an age–matched control (Braak I, left) and AD case (Braak V–VI, right). Bar=20 µm. b, Immunoblotting for PICALM, von Willebrand Factor (vWF), β3–tubulin, glial fibrillar acidic protein (GFAP), and GAPDH (loading control) in isolated microvessels and microvessel–depleted brains from controls (Braak 0–I) and AD cases (Braak V–VI). c, Relative abundance of PICALM in microvessels and microvessel–depleted brains from control and AD cases determined by densitometry analysis relative to GAPDH. Mean ± s.e.m., n=6/group; p<0.05 by ANOVA followed by Tukey’s posthoc tests. d, PICALM (green), lectin–positive endothelial capillary profiles (magenta) and microtubule–associated protein 2 (MAP2)–positive neurons (red) in the hippocampus (CA1) of an age–matched control (Braak I) and AD cases (Braak III and V–VI). Bar=20 µm. e, Quantification of PICALM–positive area (percentage) occupying lectin–positive endothelial capillary profiles in the prefrontal cortex and the CA1 hippocampal subfield. Mean ± s.d., n=9 controls (Braak 0–I) and 7 AD cases (Braak V–VI); p<0.01 by Student’s t–test. f–i, Correlations between PICALM–positive area occupying lectin–positive endothelial capillary profiles (percentage) in the prefrontal cortex and Aβ load (f), Braak stages (g), Clinical Dementia Rating (CDR) (h), and Mini–Mental State Examination (MMSE) (i). Each point in f–i is an individual value from 50 (f–g), 28 (h) and 37 (i) controls and AD cases. CDR and MMSE were not available for all cases. Significance by Pearson and Spearman rank correlation analysis.
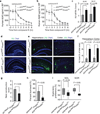
Figure 2. Diminished Aβ clearance in Picalm+/− mice
a, Immunostaining for PICALM (red) and endothelial–specific lectin (blue) in brain microvessels from Picalm+/+ and Picalm+/− mice. b, Relative abundance of PICALM protein compared to β–actin studied by immunoblotting and densitometry analysis in brain microvessels and microvessel–depleted brain homogenates in Picalm+/+ and Picalm+/− mice. P<0.05 by Student’s t–test; NS, non–significant. Means ± s.e.m. from 3–4 mice per group. c–f, Brain retention of Aβ40 (left), Aβ42 (middle) and 14C–inulin (right) (c), Aβ40 and Aβ42 clearance across the BBB (d) and by interstitial fluid (ISF) bulk flow (e) and plasma levels of Aβ40 or Aβ42 (f) after 30 min of intracerebral administration of human synthetic Aβ40, Aβ42 and 14C–inulin into the caudate nucleus of 3 month old Picalm+/+ and Picalm+/− mice. Aβ in brain and plasma was determined using human–specific Aβ40 or Aβ42 ELISA. Means ± s.e.m., n=6 mice per group. Statistical significance by Student’s t–test. NS, non–significant. p<0.05 by Student’s t–test. NS, non–significant.
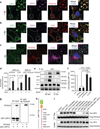
Figure 3. Diminished Aβ clearance and accelerated pathology in APPsw/0 Picalm+/− mice
a–b, ISF Aβ40 and Aβ42 levels monitored by in vivo hippocampal microdialysis of 3 month old APPsw/0; Picalm+/− and APPsw/0; Picalm+/+ mice. Baseline Aβ levels were monitored for 3 hours. c, The elimination half–life of ISF Aβ40 and Aβ42 determined after administration of compound E (γ–secretase inhibitor) 20 mg/kg intraperitoneally. d, Representative cortex and hippocampus sections stained with human Aβ specific antibodies in 3 month old APPsw/0; Picalm+/+ and APPsw/0; Picalm+/− mice, showing no Aβ deposition. Bar: 100 µm. e–f, Representative hippocampus and cortex sections stained with human Aβ specific antibodies in 9 month old APPsw/0; Picalm+/+ and APPsw/0; Picalm+/− mice, showing accelerated Aβ deposition (e) and increased Aβ load (f). In c and f, means ± s.e.m., n=5–6 mice per group. g–i, Behavioral changes in 9 month old APPsw/0; Picalm+/+ and APPsw/0; Picalm+/− mice studied by nest construction (g), burrowing (h), novel object location (NOL) and novel object recognition (NOR) (i). Means ± s.e.m. n=12–14 mice per group. In g–i, statistical significance by Student’s t–test. In i, boxplots represent the median (dark horizontal line), with the box representing the 25th and 75th percentiles, the whiskers the 5th and 95th percentile.
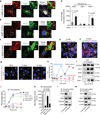
Figure 4. Endothelial specific rescue of PICALM deficiency in the hippocampus of APPsw/0 Picalm+/− mice
a, The scheme of endothelial specific rescue of PICALM in APPsw/0; Picalm+/−; Tie2–Cre mice using adeno–associated virus (AAV) carrying a Cre recombinase dependent expression cassette of Flag–Picalm transgene (see Methods for details). b, Expression of Flag–PICALM in endothelium of 5 month old APPsw/0; Picalm+/−; Tie2–Cre mouse after AAV–Flex–Picalm administration in the hippocampus. Lectin, endothelial specific marker. Co–injection of AAV–Synapsin–GFP shows insignificant expression of Flag–PICALM in neurons (< 3%). c–e, Endothelial–specific expression of PICALM in the ipsilateral hippocampus of APPsw/0; Picalm+/−; Tie2–Cre mice injected with AAV–Flex–Picalm (see b) reduces Aβ deposition (c–d) and Aβ40 and Aβ42 accumulation (e) at 6 months of age compared to the contralateral hippocampus injected with AAV–Flex control. Cortex that was not injected with AAV–Flex–Picalm shows no changes in Aβ load (c, d). Red lines in d indicate average values. P<0.01 by paired Wilcoxon Signed Rank Test. f–g, Bilateral hippocampal administration of AAV–Flex–Picalm compared to AAV–Flex (control) improves behavior in 6 month old APPsw/0; Picalm+/−; Tie2–Cre mice. Means ± s.e.m., n=10 mice per group. p<0.05 by Student’s t–test.
Figure 5. PICALM/clathrin–dependent endocytosis of Aβ–LRP1 complex by brain endothelial cells
a–b, Colocalization of LRP1–Aβ40 complex with PICALM (a) and clathrin heavy chain (CHC) (b) in human brain endothelial cells (BEC) within 30 s of FAM–Aβ40 (250 nM) treatment. c, Immunostaining for LRP1, PICALM and CHC without Aβ (– Aβ). Dapi, nuclear staining (blue). Insets: higher magnification. Bar=10 µm. d, Quantification of LRP1 puncta colocalized with PICALM in a, c and with CHC in b, c, and FAM–Aβ40 puncta colocalized with LRP1 and PICALM in a, b. Means ± s.d. from 3 primary isolates in triplicate. p<0.05 by Student’s t–test. e, Coimmunoprecipitation of PICALM, CHC and clathrin adaptor protein α–adaptin (AP–2) by LRP1–specific antibody (IP: LRP1) in BEC 30 s or 5 min after stimulation with Aβ40 (1 nM); IgG, non–immune IgG. f, LRP1 internalization in control BEC (vehicle) and after transfection with si_.Scramble_ RNA and/or si.RNAs targeting PICALM or CHC. Aβ40 (1 nM) was applied for 15 min at 4°C followed by 1 min at 37°C to initiate LRP1 internalization. Values at 4°C were taken as 100%. Means ± s.d. from 3 primary isolates in triplicate. p<0.05 by ANOVA followed by Tukey’s posthoc tests. g, In vitro binding of human recombinant PICALM to GST–tagged LRP1 C–terminus fusion protein (GST–LRP1C). h, C–terminal mutants of the human LRP1 minigene (LRP4T100). i, Coimmunoprecipitation of HA–tagged C–terminal LRP1 mutants (LRP4T100) by anti–Flag antibody (IP: Flag) in HEK293T cells after transfection with Flag–PICALM and HA–LRP4T100 mutants. HA–LRP4 and Flag–PICALM were used as loading controls.
Figure 6. PICALM associates with LRP1 during Aβ transcytosis across endothelial monolayer
a, PICALM (red), ZO–1 (green) and F–actin (magenta) in a monolayer. b, Colocalization of PICALM (red) with LRP1 (green) and FAM–Aβ40 (magenta) 1 min after LRP1–Aβ internalization at the basolateral membrane. c–d, Proximity ligation assay (PLA) depicting PICALM–LRP1 association (c, bar=10 µm) and kinetics of association between PICALM and LRP1 and clathrin and LRP1 in endothelium after Aβ40 (1 nM) addition to the basolateral membrane studied by PLA (d). e, Internalization of Aβ40 (1 nM) at the basolateral membrane of the monolayer transfected with si.LRP1, si.PICALM and si.CHC compared si_.Scramble_ or untransfected control. f–g, Basolateral–to–apical transcytosis of Aβ40 (1 nM) across endothelial monolayer transfected with si.PICALM and si.CHC compared to si_.Scramble_ (100%) over 60 min (f) and quantification of unidirectional trans–endothelial Aβ40 transport within 30 min (g). Mean ± s.e.m. from 3 primary isolates in triplicates. p<0.05 by ANOVA followed by Tukey’s posthoc test.
Figure 7. PICALM interacts with Rab5 and Rab11 during Aβ transcytosis across endothelial monolayer
a, Colocalization between PICALM (red) and Rab5 (green) in primary human brain endothelial cells (BEC) cultured with FAM–Aβ40 (250 nM) for 2 min. b, Lack of association between PICALM (red) and Rab7 (green) in BEC cultured with FAM–Aβ40 for 5 min. c, Colocalization between PICALM (red) and Rab11 (green) in BEC cultured with FAM–Aβ40 for 5 min. Dapi, nuclear staining (blue). Insets: high magnification depicting colocalization. Bar=10 µm. d, Quantification of colocalization between PICALM and Rab5, Rab7, or Rab11 puncta in a–c. e–f, Colocalization of FAM–Aβ40 (green) with Rab5 (magenta, upper) or RAB11b (magenta, bottom) 2 and 4 min after Aβ internalization at the basolateral side of endothelial monolayer, respectively. Arrows denote co–localized white puncta. g–h, PLA of PICALM–Rab11 association (g, bar=10 µm) and kinetics of PICALM association with Rab5, Rab7 and Rab11 in endothelium after addition of Aβ40 (1 nM) to the basolateral membrane (h). i, Coimmunoprecipitation of LRP1, Rab5, Rab7 and Rab11 by PICALM–specific antibody (IP: PICALM) 0, 2 and 4 min after addition of Aβ40 (1 nM) to the basloateral membrane. j–k, Basolateral–to–apical transcytosis of Aβ40 (1 nM) across monolayer expressing dominant negative Rab5–S34N, Rab7–T22N or Rab11b–S25N mutants compared to control EGFP (100%) over 60 min (j) and quantification of unidirectional Aβ40 transport within 30 min (k). Mean ± s.e.m. from 3 primary isolates in triplicates. p<0.05 by ANOVA followed by Tukey’s posthoc test. l–m, Inhibition of Rab5 (l) and Rab11 (m) GTPase activity by si.PICALM compared to si_.Scramble_ control.
Figure 8. Aβ transcytosis across AD–derived endothelial monolayer and iPSC–derived endothelium carrying the rs3851179 PICALM variants
a, qRT–PCR and western blot analysis of PICALM in control and AD endothelial monolayers. b, Diminished Aβ40 (1 nM) transcytosis across AD–derived endothelium and reversal by adenoviral–mediated Ad.PICALM re–expression. Ad.mLRP1, LRP1 minigene. Mean ± s.e.m., from 8 isolates in triplicate for control and AD monolayers. c, Diagram of CRISPR/Cas9–based generation of isogenic iPSC lines homozygous for the protective (A) or non–protective (G) allele of rs3851179. gRNA = guide RNA. d, SspI restriction digest of PCR products from iPSC genomic DNA at rs3851179 region. *denotes the CRISPR–Cas9 modified iPSC line. e, Sanger sequencing of iPSCs at rs3851179 confirming independent isogenic lines homozygous for either the G or A variant. f, FACS dot plot showing 15.7% of iPSC–derived endothelial cells via embryoid body (EB) formation are positive for endothelial markers CD31 and VE–Cadherin. g, iPSC–derived endothelial cells co–cultured with pericyte conditioned media form monolayer in vitro with ZO–1 positive tight junctions (green). Bar=100 µm. h–i, qRT–PCR and western blot analysis of PICALM (h) and Aβ40 (1 nM) transcytosis (i) in human iPSC–derived endothelial monolayers carrying the protective rs3851179 (AA) variant and the non–protective rs3851179 (GG) variant. In h–i, means ± s.e.m., from 6 cultures for each rs3851179 variant in triplicates.
Similar articles
- Anti-malaria drug artesunate prevents development of amyloid-β pathology in mice by upregulating PICALM at the blood-brain barrier.
Kisler K, Sagare AP, Lazic D, Bazzi S, Lawson E, Hsu CJ, Wang Y, Ramanathan A, Nelson AR, Zhao Z, Zlokovic BV. Kisler K, et al. Mol Neurodegener. 2023 Jan 27;18(1):7. doi: 10.1186/s13024-023-00597-5. Mol Neurodegener. 2023. PMID: 36707892 Free PMC article. - The concerted amyloid-beta clearance of LRP1 and ABCB1/P-gp across the blood-brain barrier is linked by PICALM.
Storck SE, Hartz AMS, Bernard J, Wolf A, Kachlmeier A, Mahringer A, Weggen S, Pahnke J, Pietrzik CU. Storck SE, et al. Brain Behav Immun. 2018 Oct;73:21-33. doi: 10.1016/j.bbi.2018.07.017. Epub 2018 Jul 21. Brain Behav Immun. 2018. PMID: 30041013 Free PMC article. - Magnesium Reduces Blood-Brain Barrier Permeability and Regulates Amyloid-β Transcytosis.
Zhu D, Su Y, Fu B, Xu H. Zhu D, et al. Mol Neurobiol. 2018 Sep;55(9):7118-7131. doi: 10.1007/s12035-018-0896-0. Epub 2018 Jan 30. Mol Neurobiol. 2018. PMID: 29383689 - The Role of PICALM in Alzheimer's Disease.
Xu W, Tan L, Yu JT. Xu W, et al. Mol Neurobiol. 2015 Aug;52(1):399-413. doi: 10.1007/s12035-014-8878-3. Epub 2014 Sep 4. Mol Neurobiol. 2015. PMID: 25186232 Review. - PICALM and Alzheimer's Disease: An Update and Perspectives.
Ando K, Nagaraj S, Küçükali F, de Fisenne MA, Kosa AC, Doeraene E, Lopez Gutierrez L, Brion JP, Leroy K. Ando K, et al. Cells. 2022 Dec 10;11(24):3994. doi: 10.3390/cells11243994. Cells. 2022. PMID: 36552756 Free PMC article. Review.
Cited by
- Blood-Brain Barrier Breakdown in Alzheimer's Disease: Mechanisms and Targeted Strategies.
Alkhalifa AE, Al-Ghraiybah NF, Odum J, Shunnarah JG, Austin N, Kaddoumi A. Alkhalifa AE, et al. Int J Mol Sci. 2023 Nov 14;24(22):16288. doi: 10.3390/ijms242216288. Int J Mol Sci. 2023. PMID: 38003477 Free PMC article. Review. - Tight junction modulation of the blood brain barrier: CNS delivery of small molecules.
Greene C, Campbell M. Greene C, et al. Tissue Barriers. 2016 Jan 8;4(1):e1138017. doi: 10.1080/21688370.2015.1138017. eCollection 2016 Jan-Mar. Tissue Barriers. 2016. PMID: 27141420 Free PMC article. Review. - New insights in lipid metabolism: potential therapeutic targets for the treatment of Alzheimer's disease.
Cao Y, Zhao LW, Chen ZX, Li SH. Cao Y, et al. Front Neurosci. 2024 Sep 11;18:1430465. doi: 10.3389/fnins.2024.1430465. eCollection 2024. Front Neurosci. 2024. PMID: 39323915 Free PMC article. Review. - Protective genes and pathways in Alzheimer's disease: moving towards precision interventions.
Seto M, Weiner RL, Dumitrescu L, Hohman TJ. Seto M, et al. Mol Neurodegener. 2021 Apr 29;16(1):29. doi: 10.1186/s13024-021-00452-5. Mol Neurodegener. 2021. PMID: 33926499 Free PMC article. Review. - RNA processing in neurological tissue: development, aging and disease.
Szeto RA, Tran T, Truong J, Negraes PD, Trujillo CA. Szeto RA, et al. Semin Cell Dev Biol. 2021 Jun;114:57-67. doi: 10.1016/j.semcdb.2020.09.004. Epub 2020 Oct 16. Semin Cell Dev Biol. 2021. PMID: 33077405 Free PMC article. Review.
References
- Ford MG, et al. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. - PubMed
- Marsh M, McMahon HT. The structural era of endocytosis. Science. 1999;285:215–220. - PubMed
Supplementary References
- Zlokovic BV, Mackic JB, Wang L, McComb JG, McDonough A. Differential expression of Na,K-ATPase alpha and beta subunit isoforms at the blood-brain barrier and the choroid plexus. J. Biol. Chem. 1993;268:8019–8025. - PubMed
- Wu Z, Hofman FM, Zlokovic BV. A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J. Neurosci. Methods. 2003;130:53–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37AG23084/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- SC2 NS082475/NS/NINDS NIH HHS/United States
- R00NS07743/NS/NINDS NIH HHS/United States
- R01 AG035355/AG/NIA NIH HHS/United States
- R37 NS034467/NS/NINDS NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- R01 AG039452/AG/NIA NIH HHS/United States
- R01 AG046205/AG/NIA NIH HHS/United States
- R37 AG023084/AG/NIA NIH HHS/United States
- R37NS34467/NS/NINDS NIH HHS/United States
- P01 NS074969/NS/NINDS NIH HHS/United States
- R01 AG027924/AG/NIA NIH HHS/United States
- P01 AG030128/AG/NIA NIH HHS/United States
- R01AG027924/AG/NIA NIH HHS/United States
- R01AG039452/AG/NIA NIH HHS/United States
- R01AG035355/AG/NIA NIH HHS/United States
- RF1 AG046205/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous